Baeyer’s Reagent is an alkaline solution of potassium permanganate, which is a strong oxidant. The reagent is named after Adolf Von Baeyer, a German organic chemist and Nobel laureate.
Baeyer’s reagent formula is KMnO4 (Alkaline solution).
In qualitative organic analysis, KMnO4 is used to detect the existence of unsaturation. The colour fades from purplish-pink to brown when it reacts with double or triple bonds (-C=C- or -C-C-).
Index
Baeyer’s Test
Baeyer’s test is a laboratory test used to determine the presence of a double bond in an unsaturated compound.
In this reaction, an unsaturated compound with double bonds reacts with cold and dilutes alkaline potassium permanganate to form vicinal glycols, also known as 1,2-diols. If there is unsaturation, KMnO4 is consumed, and the purple colour of potassium permanganate disappears.
Mechanism:—
MnO4– attacks the double bond to form a complex that, when hydrolyzed, yields vicinal diols.
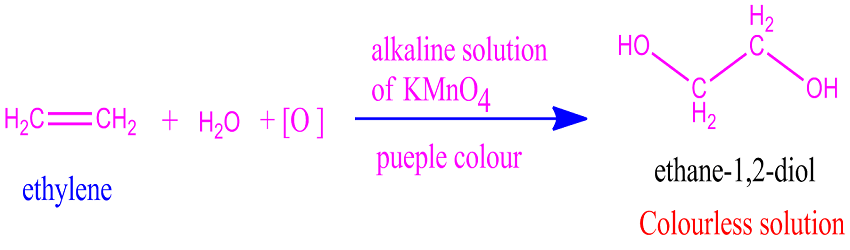
The above diagram shows how ethylene reacts with baeyer’s reagent to give ethane-1,2-diol.
Baeyer’s Reagent Examples
Here are some examples of Bayer’s reagent when it reacts with carbon-carbon double bond and triple bond compounds.
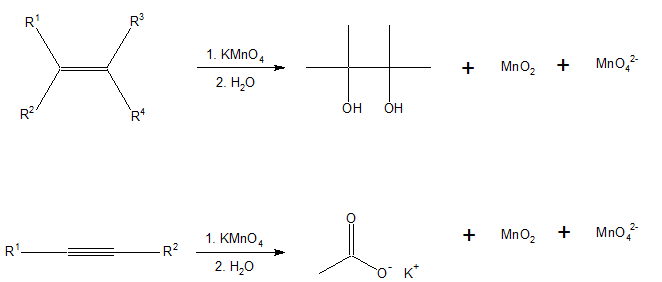
Properties
- Baeyer’s Reagent dissolves in water to produce bright pink or purple solutions. It evaporates, leaving prismatic purplish-black sparkling crystals.
- Baeyer’s Reagent is used to determine the degree of unsaturation in organic compounds.
- It is a weak oxidizing agent.
- Baeyer’s Reagent undergoes reduction, resulting in the formation of brown MnO2.
- When ethylene is treated with alkaline KMnO4 is oxidized to ethylene glycol.
- The color shift from purple (pink) KMnO4 to brown MnO2 demonstrates unsaturation.
- Aldehydes and formic acid (and formic acid ester) also pass the test.
Preparation of Baeyer’s Reagent
- Dissolve 1 gram of solid KMnO4 in 100 mL of distilled water to generate a 1% potassium permanganate solution.
- The container with the stoppered lid is filled with 10 grams of anhydrous sodium carbonate (Na2CO3), which is shaken until completely dissolved.
- Storing the solution in the dark and keeping it cool keeps it fresh
FAQs
The Baeyer test uses dilute Potassium Permanganate to oxidize the carbon-carbon double or triple bond. The process is referred to as oxidation because the double bond is replaced by a hydroxyl group (an OH group). Carbon’s charge shifts from +1 to +2, resulting in the loss of an electron (and is thus oxidized).
No, they aren’t the same tests, but both are used to find the unsaturation of the given compound.
Related Articles
