The Gabriel Phthalimide Synthesis is a chemical reaction, commonly used to convert primary alkyl halides into primary amines.
In 1887, Siegmund Gabriel, a German chemist, along with his partner James Dornbush, made the discovery of the Gabriel Synthesis.
Gabriel Synthesis is a chemical reaction that has since become a widely used method for the preparation of primary amines.
Index
Explanation:
Gabriel Phthalimide Synthesis is a chemical reaction that is used to prepare primary amines from primary alkyl halides.
The Gabriel Pthalamide synthesis has several advantages over other methods of primary amine synthesis. It is a simple and efficient method that can be used to synthesize a wide range of primary amines from readily available starting materials.
The reaction also proceeds under mild conditions and does not require the use of harsh reagents or elevated temperatures.
One of the benefits of using this reaction is that it avoids the need for over alkylation.
Though a limitation is that this method cannot be used to prepare other amines (i.e secondary or tertiary amines).
Mechanism:
The reaction mechanism can be mainly divided into three main steps:
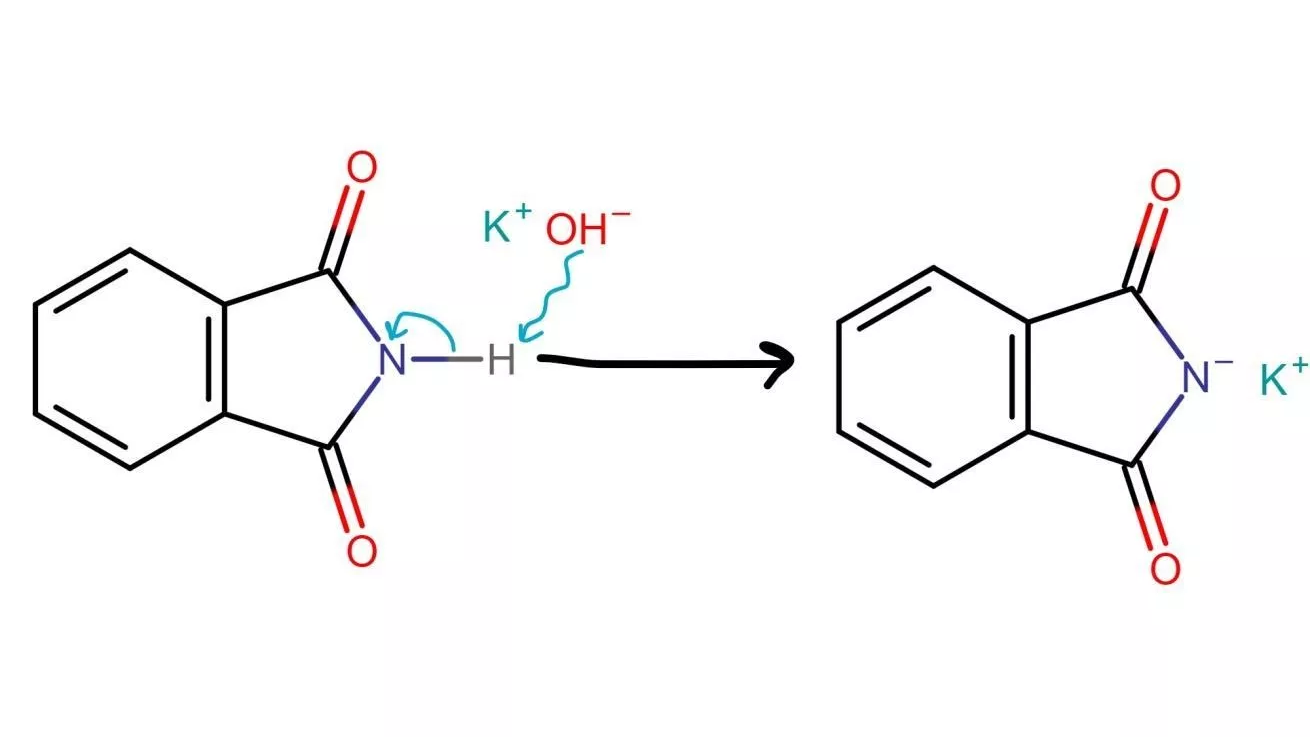
1. First step involves the addition of potassium hydroxide to phthalimide results in an acid-base reaction, where the hydroxide ion removes a hydrogen atom from the imide, resulting in the formation of a more acidic proton compared to a simple amine.
This increased acidity is due to the presence of two adjacent carbonyl-like groups in the imide, which provide resonance stabilization. As a result, the resulting imide ion serves as a potent nucleophile.
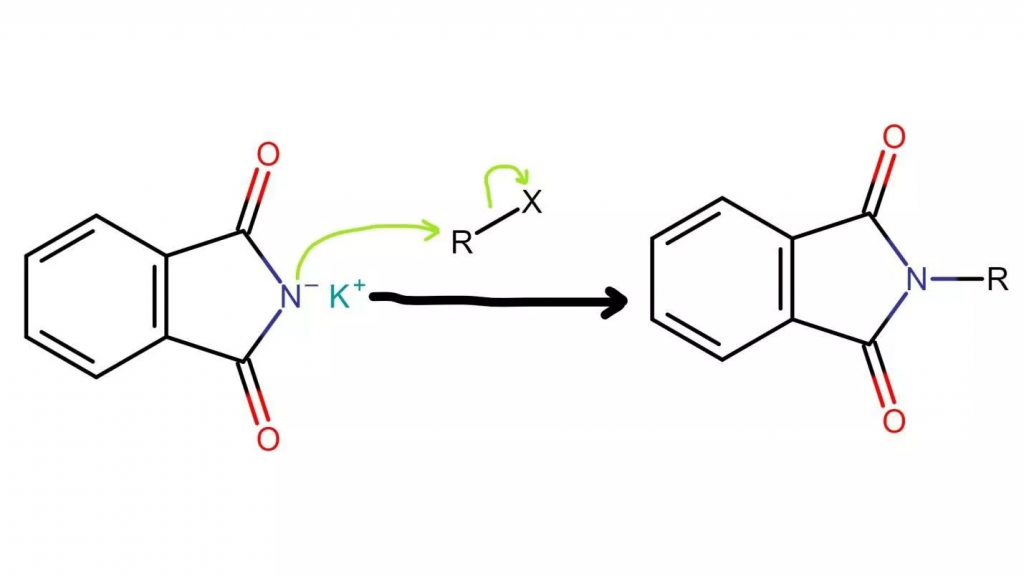
2. In the second step of the Gabriel synthesis, an N-alkyl phthalimide is formed. This involves the attack of the electrophilic carbon in the alkyl halide by the nucleophilic imide ion, resulting in the replacement of the halogen atom (fluorine, chlorine, bromine, or iodine) with the nitrogen atom.
As a result of this reaction, the nitrogen atom becomes bonded to the carbon atom, leading to the formation of an N-alkyl phthalimide.
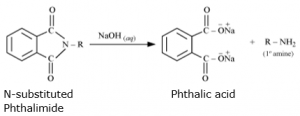
In the third step, the R group binds with the nitrogen atom, resembling the process of base-catalyzed hydrolysis of esters, except that the resulting bond is between nitrogen and the R group instead of oxygen and nitrogen.
The carbon atom is then targeted by the hydroxide ion present in potassium hydroxide, which causes the N-alkyl phthalimide to break apart and makes a primary amine.
Applications:
- Gabriel Phthalimide Synthesis is used in the preparation of primary amines.
- This Gabriel Phthalimisde Synthesis reaction has been widely applied for sulfonamide and imide alkylation, as well as for the deprotection (process of removing a protecting group) of these compounds to yield amines.
FAQ’S
It doesn’t give secondary and tertiary amine as a product.
It cannot be used to prepare aryl amines also.
Reagents required for gabriel phthalimide synthesis are potassium phthalimide, alkyl halide, or aryl halide and a strong base such as potassium hydroxide.
By Gabriel phthalimide synthesis, because aryl halides do not undergo nucleophilic substitution with the anion formed from phthalimide, which is a key intermediate in this synthesis.
It is a chemical process commonly used to convert primary alkyl halides into primary amines.
N-potassium phthalimide is used as a source of the nitrogen atom.
Related Topics
