Tollens Reagent is a chemical compound used for distinguishing between aldehydes and ketone functional groups along with some alpha-hydroxy ketones. This compound has the chemical formula Ag(NH3)2OH.
Index
More About Tollens Reagent
Tollen’s reagent consists of a solution of Silver Nitrate(AgNO3), Ammonia(NH3), and Sodium Hydroxide(NaOH). It was discovered by the German Chemist Bernhard Tollens, and thus the name.
Tollens Reagent Preparation
Due to its short shelf life, this reagent is not available commercially. It is prepared freshly in the laboratory. The preparation of Tollens reagent takes two steps.
Step 1: A few drops of dilute sodium hydroxide are added to aqueous 0.1 M silver nitrate.
2AgNO3 + 2NaOH → Ag2O + 2NaNO3 + H2O
The above reaction precipitates a brown solid, silver(I) oxide.
Step 2: Next, an adequate amount of aqueous ammonia is added to dissolve brown silver(I) oxide.
Ag2O + 4NH3 + 2NaOH + H2O → 2[Ag(NH3)2]OH
The resulting solution contains [Ag(NH3)2]+ in the mixture, which is the main component of Tollens reagent.
Another preparation method is to add aqueous ammonia directly to the silver nitrate solution. Initially, ammonia will induce the formation of silver oxide, but upon adding more ammonia, this solid precipitate dissolves to give a clear solution of [Ag(NH3)2]+.
Tollens Reagent Test
One of the famous application of this reagent is Tollen’s test where aldehyde gives a grey-black precipitate or a silver mirror upon the addition of freshly prepared Tollens reagent to the solution.
Tollens Test Procedure
- A small amount(50mg) of the given compound is dissolved in aldehyde-free alcohol(2ml).
- Next, freshly prepared Tollen’s reagent is added to the solution and it is warmed in a hot water bath.
- The presence of an aldehyde is confirmed by the appearance of a grey-black precipitate or a silver mirror.
This reaction is a redox reaction as the silver ion goes from +1 oxidation state to 0 oxidation state. Since the reaction occurs in an alkaline medium, the carboxylic acid isn’t obtained directly. Instead, a carboxylate ion is obtained. This precipitates out silver, thus forming the silver mirror on the edges or the walls of the test tube.
Alpha Hydroxy Ketone Tollens Test
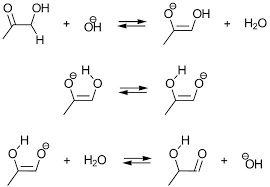
Alpha hydroxy ketones have the ability to tautomerize aldehydes and as aldehydes give Tollens test, alpha-hydroxy ketones are also able give Tollen’s test.
FAQs
Tollens reagent is a chemical compound used to distinguish between aldehydes and ketones. This reagent has the chemical formula Ag(NH3)2OH.
Tollens test is a qualitative laboratory test, used to distinguish between an aldehyde and a ketone. It is based on the fact that aldehydes are readily oxidized whereas ketones are not.
Tollen’s reagent consists of silver nitrate(AgNO3), ammonia(NH3), and sodium hydroxide(NaOH).
Related Articles
