Hinsberg reagent or benzene sulfonyl chloride is a reagent utilized in the Hinsberg test to differentiate primary, secondary, and tertiary amines present in a given sample.
Hinsberg Reagents formula is C6H5SO2Cl
Hinsberg reagent was developed in the late 19th century. It is named so as it is used in a reaction called the Hinsberg reaction.
And the Hinsberg reaction was first described by Oscar Hinsberg in 1890.
Index
Explanation
Benzene sulfonyl chloride, which is commonly referred to as the Hinsberg reagent, is used in the Hinsberg test to identify and differentiate primary, secondary, and tertiary amines in a sample.
This reagent is an organosulfur compound with a sulphur base which has the chemical formula (C6H5SO2Cl) and appears as a viscous, colourless oil that can dissolve in organic solvents.
The Hinsberg reagent reacts with compounds that have reactive O-H and N-H bonds and is utilized in the production of sulfonamides and sulfonamide esters through its reaction with amines and alcohols, respectively.
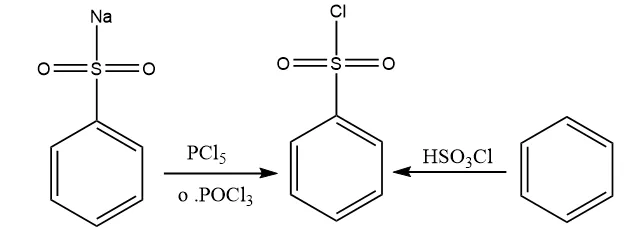
Preparation
The Hinsberg Reagent can be obtained through two methods:
- The first method involves the chlorination of benzene sulfonic acid or its salts using phosphorus oxychloride (POCl3).
- The second method involves the reaction of benzene with chlorosulfuric acid (HSO3Cl) to produce the required reagent.
Hinsberg Test
The Hinsberg test, was developed by German chemist Oscar Heinrich Daniel Hinsberg in 1890. It utilizes a chemical reaction with the reagent to distinguish between primary, secondary, and tertiary amines.
In this test, the amines behave as nucleophiles and attack the electrophilic sulfonyl chloride. It leads to the displacement of the chloride and the formation of sulfonamides.
When a mixture of primary and secondary amines is used, sulfonamides are produced, which are insoluble and precipitate out of the solution as a solid.
Principle
The Hinsberg test is used to identify primary and secondary amines by reacting them with benzene sulfonyl chloride to form sulfonamides.
Tertiary amines do not form stable sulfonamides, so any product formed must be a primary or secondary amine.
If the resulting sulfonamide is soluble in aqueous sodium hydroxide, it indicates a primary amine.
Whereas, if it is insoluble in both aqueous sodium hydroxide and hydrogen chloride, it indicates a secondary amine.
The sulfonamide of a primary amine is soluble in an aqueous base because of the presence of an acidic hydrogen atom on the nitrogen, which reacts with sodium hydroxide to form a sulfonamide sodium salt.
When benzene sulfonyl chloride is mixed with a tertiary amine, no reaction takes place, and instead, an oil or insoluble solid is formed. However, upon the addition of hydrogen chloride, the resulting mixture becomes clear as the unreacted amine dissolves, forming an amine salt.
Mechanism
Initially, the amine undergoes an addition-elimination reaction with benzene sulfonyl chloride, which is highly electrophilic.
Subsequently, in the presence of sodium hydroxide, the chlorine and one proton from the amine are eliminated in a stepwise manner, resulting in the formation of a sulfonamide salt of sodium.
Procedure
Firstly, in a large test tube, add around 8-10 drops of amine, followed by 10 drops of benzene sulfonyl chloride, and 10 mL of NaOH with a concentration of 10%. Vigorously shake the mixture using a cork.
After mixing, observe the solution to determine if there is a single layer (indicating the presence of a primary amine) or a double layer (indicating the presence of a secondary amine).
If there is a double layer, use a separatory funnel to separate the bottom aqueous layer from the organic layer. Check if the organic layer is soluble in 5% HCl. If the organic layer is insoluble, it suggests the presence of a secondary amine.
Reaction Pathways
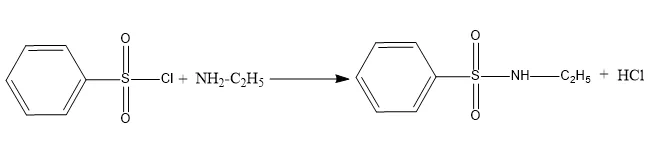
A reaction between primary amines and benzene sulfonyl chloride results in the formation of an alkali-soluble sulfonamide product.
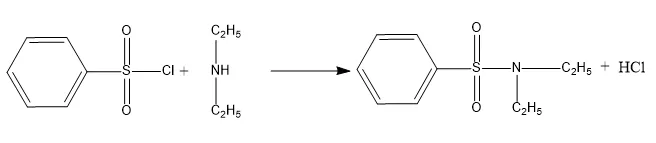
The reaction between benzene sulfonyl chloride and secondary amines results in the formation of a sulfonamide product that is insoluble in alkali.
When tertiary amine is combined with benzene sulfonyl chloride reagent (hinsberg’s reagent), no reaction takes place.
However, when tertiary amines are present, the hydrolysis of sulfonyl chloride is facilitated. The resulting salts are soluble in water.
Hinsberg’s reagent reacts differently with primary, secondary, and tertiary amines, leading to different sulfonamide products with varying solubility in alkali.
Applications
- Hinsberg Reagent is used to discriminate between 1°, 2°, and 3° amines.
- Hinsberg Reagent is used in the synthesis of amines as well.
FAQ’S
Benzene sulfonyl chloride, also known as the Hinsberg reagent, is an organosulfur compound with the chemical formula C6H5SO2Cl used to differentiate between primary, secondary and tertiary amines.
It has a dense, colourless oily texture and is readily soluble in organic solvents.
Benzene Sulphonyl Chloride.
No, tertiary amines do not react with the Hinsberg reagent.
C6H5SO2Cl is the chemical formula of the Hinsberg Reagent.
Related Topics
