The Frenkel defect, also known as the Frenkel pair was named after the Russian physicist Yakov Frenkel, who first described it in 1926.
Frenkel discovered this while studying the electrical conductivity of ionic crystals. He noticed that the conductivity of these crystals was higher than what would be expected based on the concentration of mobile ions alone.
Frenkel realized that the extra conductivity was due to the movement of ions from their normal lattice sites to interstitial sites. This created both a vacancy and an interstitial site.
Index
Explanation
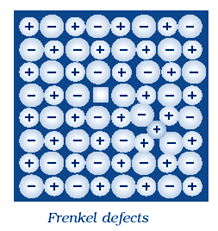
A Frenkel defect is a type of point defect in a crystal lattice. Here an ion is displaced from its normal lattice site and moves to an interstitial site. Creating a vacancy at its original position.
In other words, the ion “jumps” from its normal position to a nearby empty space within the crystal, leaving behind a gap or vacancy where it was originally located.
It typically occurs in materials where the anion (negatively charged ions) is larger than the cation (positively charged ions).
When such a material is subjected to high temperatures or radiation, cations can be displaced from their lattice sites, creating a vacancy. The displaced cation can then move into an interstitial site, creating a Frenkel pair.
It is often seen in ionic compounds, such as metal halides, where the anions are much larger than the cations.
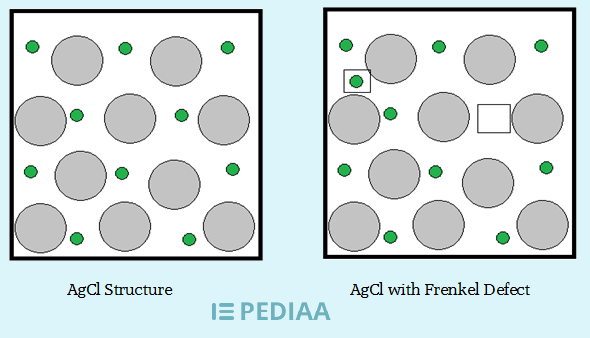
For example, silver halides, which are used in photographic film, exhibit the this defect when exposed to light.
The light creates electron-hole pairs, which cause silver ions to be displaced and create Frenkel pairs. Which were shown in the image below.
Applications:
- Solid-state lighting: Frenkel defects are used to create solid-state lighting in materials such as gallium nitride (GaN) and zinc oxide (ZnO).
- Ionic conductors: Frenkel defect can create ionic conductivity in solid-state materials. It facilitates the formation of lithium-rich and lithium-vacancy couples that can be used in applications such as solid-state batteries.
- Conductivity: The solids having Frenkel defect show conductivity and diffusion in the solid state due to the presence of vacant lattice sites, which is important in thermoelectric applications.
Related topics
Schottky Defect
FAQ’S
KBr (Potassium Bromide)
No, it has no effect on the density of a crystal.
AgBr (Silver Bromide)
It is also known as a dislocation defect because atoms present in the crystal lattice are dislocated to an interstitial site in this defect.
