SN1 Reaction is a Nucleophilic Substitution reaction (A class of reactions wherein the electron-rich nucleophile attacks a positively charged electrophile to replace a leaving group) in which the rate-determining step involves 1 component. SN1 reactions are unimolecular and proceed through an intermediate carbocation. The first step, being slower, determines the rate.
Index
What is SN1 Reaction?
The SN1 reaction is a substitution reaction, the name of which refers to the Hughes-Ingold symbol of the mechanism. “SN” stands for “substitution nucleophilic“, and the “1” says that the rate-determining step is unimolecular.
The organic reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols.
This type of mechanism involves two steps.
1. The first step is the ionization of alkyl halide in the presence of aqueous acetone or ethyl alcohol
2. The second step is the attack of the nucleophile.
SN1 Reaction Mechanism
To understand the mechanism of SN1 reactions, let us take the example of hydrolysis of tertiary butyl bromide
- Formation of a tert-butyl carbocation by separation of a leaving group (a bromide anion) from the carbon atom. This step is slow.
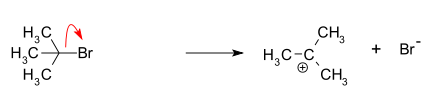
- Nucleophilic attack: the carbocation reacts with the nucleophile. If the nucleophile is a neutral molecule (i.e. a solvent) a third step is required to complete the reaction. When the solvent is water, the intermediate is an oxonium ion. This reaction step is fast.

- Deprotonation: Removal of a proton on the protonated nucleophile by water acting as a base, forming the alcohol and a hydronium ion. This reaction step is fast.
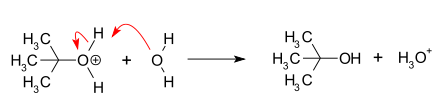
Characteristics of SN1 Reaction
- This is a two-step reaction process.
- Carbon-halogen bond breaks which result in a positively charged carbon (carbocation intermediate).
- Nucleophile attacks the carbocation and forms a new bond.
- Unimolecular and follows first-order kinetics
- Rate of the reaction depends on the concentration of the substrate (alkyl halide)
- Has a racemization stereochemistry, i.e., both retention and inversion products are formed
- A polar protic solvent is used to enhance the reactivity
Stereochemistry of SN1 Reaction
If we start with an enantiomerically pure product, (that is, one enantiomer), these reactions tend to result in a mixture of products where the stereochemistry is the same as the starting material (retention) or opposite (inversion). In other words, some degree of racemization will take place.
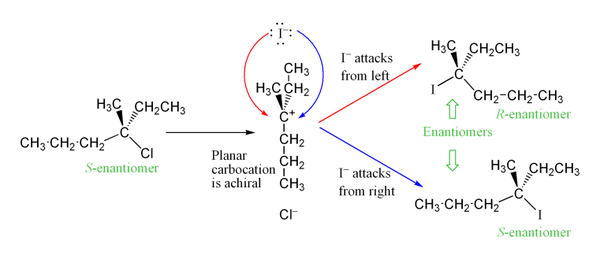
However, an excess of one stereoisomer can be observed, as the leaving group can remain in proximity to the carbocation intermediate for a short time and block the nucleophilic attack. This stands in contrast to the SN2 mechanism, which is a stereospecific mechanism where stereochemistry is always inverted as the nucleophile comes in from the rear side of the leaving group.
Factors Affecting SN1 Reaction Mechanism
- The reaction is favoured by a highly substituted alkyl halide and ideally the one which will not lead to rearrangement
- A non-basic nucleophile is preferred (to reduce the elimination E1 side reaction)
- A good leaving group is preferred (like Iodine or Bromine)
- Polar protic solvents are most effective and suitable ones for the reaction.
Examples
Question 1. Rank the following by increasing reactivity in an SN1 reaction.
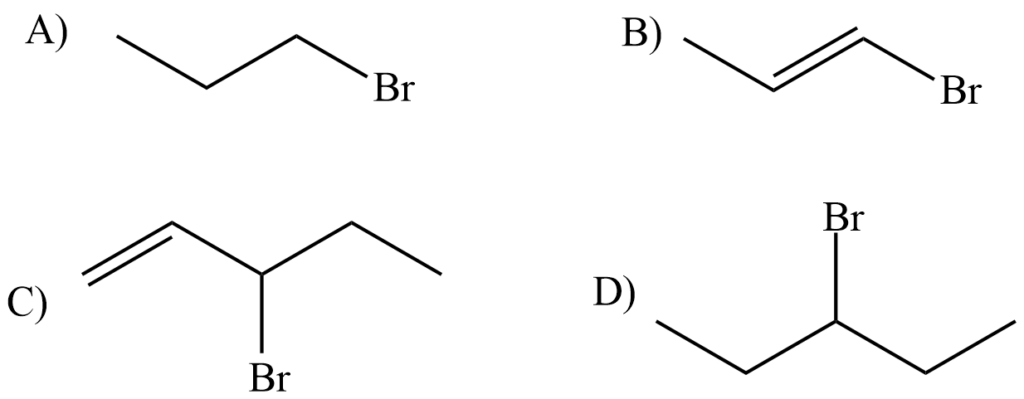
Solution. By considering the stability of the intermediate carbocation.
A < D < B < C (most reactive)
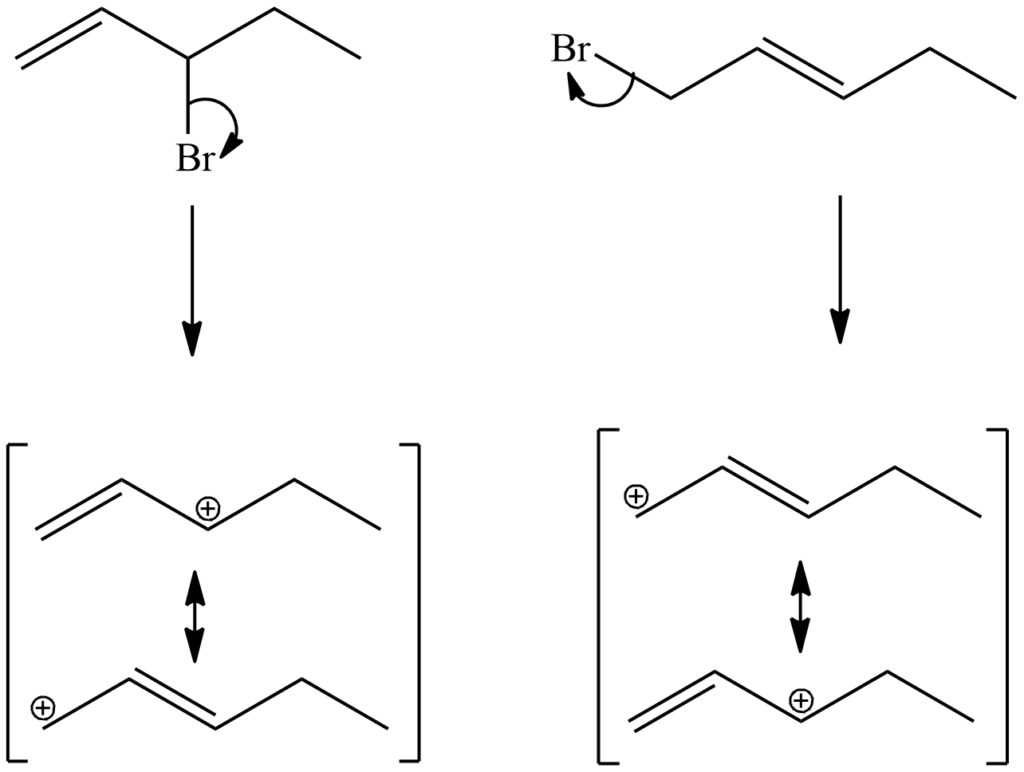
Question 2. 3-bromo-1-pentene and 1-bromo-2-pentene undergo SN1 reaction at almost the same rate, but one is a secondary halide while the other is a primary halide. Explain why?
Solution. They both have the same intermediate cations in their resonance forms.
FAQs
If a primary haloalkane did use this mechanism, a primary carbocation would be formed, which is much more energetically unstable than the tertiary one formed from tertiary haloalkanes. Therefore, it is much more challenging to produce.
The SN1 tends to proceed in polar protic solvents. The SN2 reaction is favored by polar aprotic solvents – these are solvents such as acetone, DMSO, acetonitrile, or DMF that are polar enough to dissolve the substrate and nucleophile but do not participate in hydrogen bonding with the nucleophile.
In an SN1 reaction, the rate determining step is the loss of the leaving group to form the intermediate carbocation. The more stable the carbocation is, the easier it is to form, and the faster the SN1 reaction will be.

Pingback: Swarts Reaction - Mechanism, Application & Example | ProtonsTalk