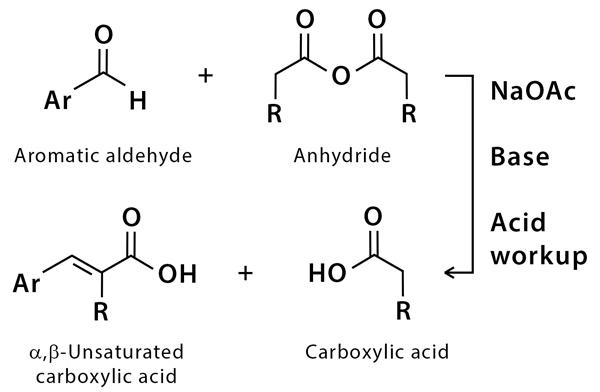
The Perkin Reaction involves the synthesis of \(\alpha, \beta\)-unsaturated acids. It is an aldol type condensation reaction between an aromatic aldehyde (Ar-CHO) and a carboxylic acid anhydride (RCO)2O catalyzed by a carboxylate ion (the potassium or the sodium salt of the carboxylic acid, RCOOK or RCOONa).
Index
History
In 1868, a British chemist, William H. Perkin, described the synthesis of coumarin by heating the sodium salt of salicylaldehyde and acetic anhydride. Further study into the reaction led to the key discovery of a new method to synthesize cinnamic acid. This basic reaction then came to be known as the perkin reaction.
Perkin Reaction Mechanism
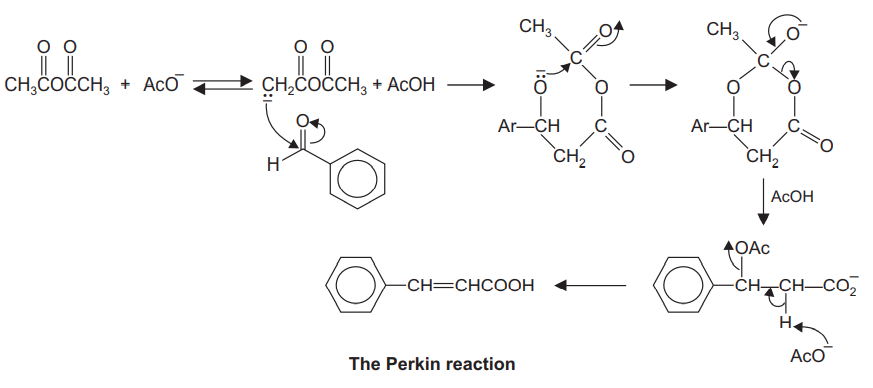
Perkin reaction can be regarded as essentially the condensation of a carbonyl compound ‘A’ and an acid anhydride salt ‘B’. In the resulting acids, the substituents in the carbonyl ‘A’ appear in the \(\beta\) position, and those in the acid component appear in the \(\alpha\) position.
Step 1: Deprotonation of anhydride. The \(\alpha\) carbon of the anhydride has acidic protons. Such carbons are called active methylated carbons.
Step 2: Nucleophilic attack on carbonyl compound by an anhydride. The carbonyl carbon of the carbonyl compound is electrophilic and often reacts with nucleophilic (deprotonated anhydride).
Step 3: Formation of 6-membered intermediate.
Step 4: Ring-opening to form carboxylate ion.
Step 5: Formation of \(\alpha, \beta\)-unsaturated carboxylic acid.
Applications of Perkin Reaction
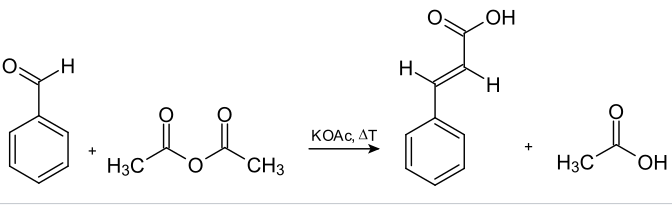
The most common use for the perkin reaction is the production of cinnamic acid and its derivatives. Benzaldehyde reacts with acetic anhydride in the presence of potassium acetate and heat to produce cinnamic acid and acetic acid. This reaction is often involved in the industrial preparation of cinnamic acid.
FAQs
Perkin reaction involves the synthesis of α, β-unsaturated acids. It is an aldol-type condensation reaction between an aromatic aldehyde (Ar-CHO) and a carboxylic acid anhydride (RCO)2O catalyzed by a carboxylate ion (the potassium or sodium salt of the carboxylic acid, RCOOK, or RCOONa).
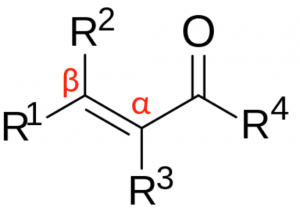 (Source)
(Source)
In the figure, we show the structure of a general carbonyl compound with a double bond. When R4 is a Hydrogen atom, the compound is a carboxylic acid. A compound is said to be saturated when it has only C-C single bonds. The compound is saturated with the maximum number of hydrogen atoms it can bond with.
Here we have a double bond, therefore, the compound is unsaturated. The first carbon bonded to the carbon atom is said to be the \(\alpha\) position. The carbon bonded to this \(\alpha\) carbon is called the \(\beta\) carbon. Here R3 is the \(\alpha\) bonded substituent, and R4 is the \(\beta\) bonded substituent. Therefore, this compound is called an \(\alpha, \beta\)-unsaturated carboxylic acid.
The alkali salt of the acid involved in the reaction is used as the base catalyst but other bases can also be used instead.
More Organic Reactions
