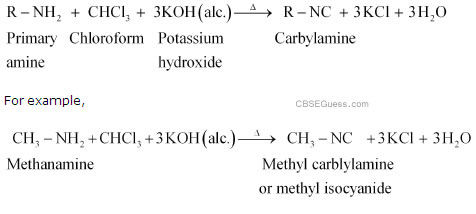
The Carbylamine Reaction (also called the Hofmann Isocyanide Synthesis) is a reaction which produces an isocyanide, starting from a primary amine, chloroform and a base.
Index
More About Carbylamine Reaction
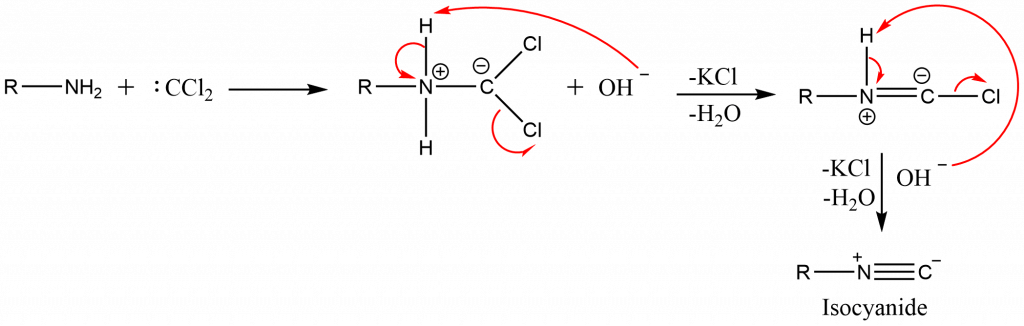
This reaction involves the formation of the very highly reactive carbene intermediate, namely dichlorocarbene (which is electrophilic), which can react with the Nitrogen atom of the primary amine (which is nucleophilic).
The nucleophilicity of the Nitrogen atom of the primary amine can vary based upon the alkyl/aryl group attached to it (and also by the effect of the solvent being used).
History
The reaction was done by Hofmann (treatment of aniline with chloroform in potassium hydroxide) and was one of the first reactions which led to the discovery of isocyanides (as being isomers of cyanides).
Gautier also independently observed the formation of isocyanides in the reaction of silver cyanide with alkyl iodides.
Carbylamine Reaction Mechanism
Step 1: Deprotonation of chloroform’s Hydrogen by the base.
Step 2: Formation of dichlorocarbene by elimination of chlorine.
Step 3: Electrophilic attack by dichlorocarbene on the Nitrogen of the primary amine.
Step 4: Two successive dehydrohalogenations to form isocyanide.
For the dehydrohalogenation to occur in step 4, there must be 2 or more Hydrogen atoms present. Hence, this reaction does not occur for secondary and tertiary amines.
For this reason, the carbylamine reaction is used as an effective identification test for primary amines. The sample is heated with potassium hydroxide (alcoholic) and chloroform. The formation of isocyanide is detected immediately by a distinctive foul smell. This way, primary amines can be differentiated from secondary and tertiary amines.
This reaction was initially one of the very few methods to synthesise isocyanides. Now more reliable synthetic routes have been discovered, such as dehydration of formamides, phase-transfer catalysis (PTC) method, using benzyltriethylammonium chloride as the phase-transfer catalyst, etc.
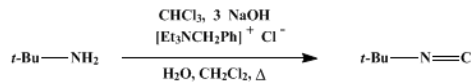
Applications
The two main applications of this reaction are:
- Synthesis of isocyanides.
- Detection of primary amines.
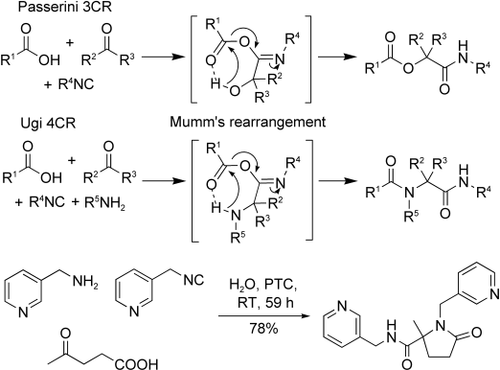
The reaction was one of the first steps towards learning the chemistry of isocyanides. Now with more reliable methods of isocyanide synthesis, isocyanides have had a wide range of uses. Isocyanides are involved in:
- Multicomponent Reactions (MCRs)
- Transition-metal-catalysed insertions
- Polymerization reactions
And most importantly,
- Synthesis of heterocycles (indoles, pyrroles, etc)
FAQs
Carbylamine reaction (or Hofmann’s isocyanide synthesis) is a method of preparation of isocyanides/isonitriles from primary amines by the reaction of a primary amine, chloroform and a base.
The following is an example this reaction: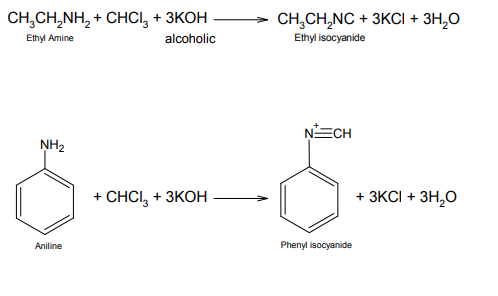
Fig. Preparation of Ethyl Isocyanide from Ethyl Amine (Source)
Ethyl amine is a primary amine and hence can undergo carbylamine reaction. Dichlorocarbene is formed from chloroform after deprotonation and loss of Chloroform. The electrophilic dichlorocarbene then adds to the Nitrogen (nucleophilic) of ethyl amine, followed by two successive dehydrochlorinations to form ethyl isocyanide.
More Organic Reactions
