Cathode Ray Experiment or also commonly known as J.J. Thomson’s experiment is one of the important experiments in particle physics. Electrons which are one of the fundamental particles were discovered through this experiment.
Index
History
Cathode rays were first identified by a German physicist named Johann Hittorf when he realized that something was travelling through the tube. Eugene Goldstein was the one who actually gave cathode rays their name.
Scientists came up with two theories regarding cathode rays when they were originally discovered.
Scientists Crooks and Arthur Schuster said they were electrically charged atoms. German scientists Eilhard Wiedemann, Heinrich Hertz and Goldstein said they were some new form of electromagnetic radiation.
This debate was resolved when J.J. Thomson measured the weight of cathode rays and showed that they were actually a beam of particles. They were later named electrons after particles postulated by George Johnstone Stoney.
The Experiment
When a high voltage is applied between the two electrodes of an evacuated discharge tube and the back of anode of the discharge tube is coated with a material, like zinc sulfide. When cathode rays hit from the cathode travel towards the anode and hit the anode, a fluorescence or glow is produced.
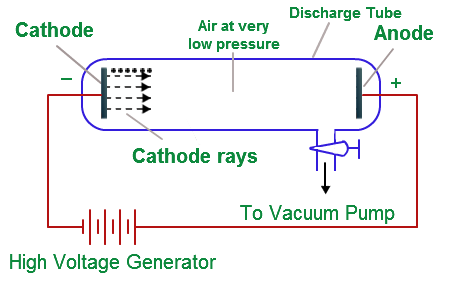
If a gas is pumped into the cathode ray tube, the electrons excite the gas molecules and the gas starts to glow. The same phenomenon is also responsible for the glowing of the fluorescence material.
There are also a lot of modified experiments done based on the original one.
Placing a Solid Object
If an object is placed between in the path of the cathode rays, its shadow is formed on the surface behind anode.
From this scientists concluded that cathode rays travel in straight lines.

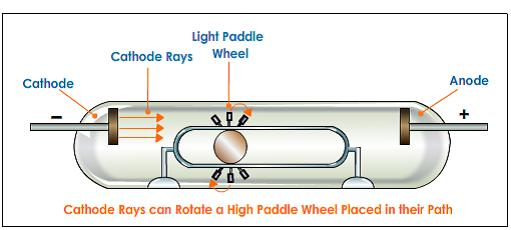
Placing a Rotatable Object
A very light fan-like rotatable object is placed in the path of the cathode rays. Using this, scientists observed that cathode rays have mass because the fan rotates when the cathode rays are produced and hits the rotatable object.
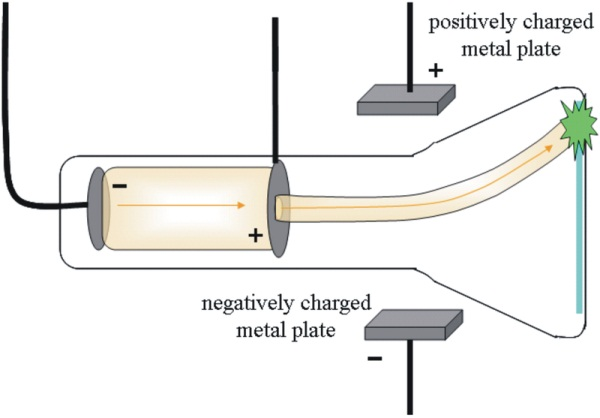
Applying an Electric Field
Cathode rays deviate from their straight path due to the application of an electric field perpendicular to it.
From this experiment, scientists calculated the magnitude and type of charge on the cathode ray particles (i.e., electrons).
Stopping Potential
When we apply electric field in parallel but in the opposite direction to the cathode rays and if it is sufficiently high for the cathode rays to stop, then the magnitude of the applied voltage is called stopping potential.
\(eV = \frac{1}{2}mv^2\);
here,
\(V\) = stopping potential,
\(e\) = magnitude of charge of electron
\(v\) = velocity of electron
\(m\) = mass of electron.
When electrons move from one point to another, say from A to B. At point B the electron stops due to the activation of stopping potential, so we apply the law of conservation of energy between the two points.
Hence, energy of electron at point A = energy of electron at point B
Applying a Magnetic Field
Cathode rays also get deflected from their path if a magnetic field is applied. This also helped scientists in finding the charge of electrons.
The above modified experiments were performed by J.J. Thomson in order to find electrons. So, the cathode ray experiment is also commonly known as J.J. Thomson’s experiment.
Applications
1. In old displays, vacuum tubes were used by directing the beam of electrons using deflection plates, then the beam causes fluorescence on the screen which we see as white.
2. Vacuum tubes were initially used in the place of silicon transistors in electronics. They helped the transition from the industrial age to the digital.
3. Used to measure changes in electrical voltage with time. The device is called an oscilloscope which is frequently used in medical treatment.
4. The cathode ray amusement device was the world’s first video game. The cathode ray tube produces a ray of light, whose path must be adjusted so that it hits the targets printed on the screen.

5. These were used in old military radar screens.
Example Problem
Question. The charge of an electron \(e = 1.602 * 10^{-19}\) and its is mass \(m= 9.11 * 10^{-31}\). Let the stopping potential of an electron travelling in a cathode ray tube be \(V = 5V\), then find the velocity of an electron travelling (charge of an electron \(e = 1.602 * 10^{-19}\) and mass \(m= 9.11 8 10^{-31}\)).
Answer. Here we need to find the velocity of travelling electrons using the given stopping potential.
We know that \(eV = \frac{1}{2}mv^2\), the charge(e) and mass(m) of electron are also given as,
\(e = 1.602 * 10^{-19}\) and \(m= 9.11 * 10^{-31}\)
Now substituting the values of \(e, m, V\).
\((1.602 * 10^{-19})(5) = \frac{1}{2}(9.11 * 10^{-31})(v^2)\) \(v^2 = \frac{(1.602 * 10^{-19})(5)(2)}{9.11 * 10^{-31}}\) \(v = 1.33 * 10^{6} m/s\)FAQs
A cathode ray tube is a vacuum tube with a fluorescence material at the back of the anode. They both are essentially the same except that the cathode ray tube’s end glows when turned on. Hence, a cathode ray tube and a vacuum tube are different in the way they are used in the real world.
J.J. Thomson first constructed a cathode ray tube with a cylinder on one side with two slits in it, leading the beam to electrometers which could measure small electric charges.
That’s all about Cathode Ray Experiment.
How do you feel about this article? Comment down below and let us know your thoughts.
