Sodium Metabisulfite (Na2S2O5), also known as Sodium Pyrosulfite, is an inorganic compound, with a chemical formula Na2S2O5. It is also known as Disodium Metabisulfite.
Index
Structure & Formula
The chemical formula for sodium metabisulfite is Na2S2O5.
Structure of Sodium Metabisulfite is as follows:
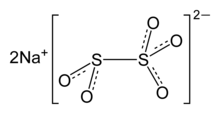
The anion metabisulfite consists of an SO2 group linked to an SO3 group. The negative charge is more localised on the SO3 end.
Properties of Sodium Metabisulfite
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
The compound has the following physical properties:
| Color | White/whitish-yellow |
| Smell | Faintly pungent smell |
| Solubility | Fairly soluble in water, highly soluble in glycerol, not very soluble in ethanol |
| Melting Point | 170℃ |
| Boiling Point | Decomposes |

Chemical Properties
The inorganic compound has the following chemical properties:
- Chemical formula – Na2S2O5.
- Molar mass – 190.107 g/mole
- Density – 1.48 g/cm3
- When introduced to water, the compound liberates sulfur dioxide gas which has a very pungent and unpleasant odor.
- When heated, the compound undergoes decomposition to form sodium sulfite and sulfur dioxide.
Production of Sodium Metabisulfite
The coumpound can be prepared and produced by treating a solution of sodium hydroxide with sulfur dioxide.
SO2 + 2NaOH → Na2SO3 + H2O
SO2 + Na2SO3 → Na2S2O5
The following reactions yield a final product of colorless solid Na2S2O5.
Applications of Sodium Metabisulfite
- The compound is one of the primary ingredients used in Campden tablets.
- It is also used to sanitize equipment that is used for wine-making.
- The inorganic compound is used in photography.
- It is used as a rust-stain remover when combined with sodium hydrosulfite.
- It is used as a bleaching agent in the production of coconut cream, as a reducing agent to break sulfide bonds in shrunken items of clothing made of natural fibres.
- It is used to preserve fruits during shipping, as a solvent in the extraction of starch from fruit and cereal crops.
FAQs
The inorganic compound is a reducing agent used for the preservation of fresh and dried fruits, vegetables and wines. It has been proven to be safe, with no side effects, and approved by U.S. Food and Drug Administration(FDA) and European Food Safety Authority(EFSA).
The Cosmetic Ingredient Review Expert Panel had assessed the safety of the inorganic compound and had concluded that this compound is safe for its indicated uses in cosmetics and skincare products. This may not be the case for people who are allergic to sulfites.
Foods such as jams, soup mixes, baked goods, pickled items, potato chips etc., contain sodium metabisulfite.
More on Inorganic Compounds
