Copper hydroxide (Cu(OH)2) otherwise represented as copper(II) hydroxide or cupric hydroxide is an inorganic compound that is a crystalline solid. It is usually a bluish-green solid or a pale greenish-blue solid. It is a strong base, but its action is difficult to detect due to its low solubility in water.
Index
History
Alchemists were the ones who first produced Cu(OH)2 from copper sulfate(blue vitriol) and NaOH or KOH(Iye) solutions. Although copper hydroxide was known to humanity since around 5000 BC when copper smelting began.
The compound was commercially produced when it began to be used in pigments for ceramics and painting. The commercial production was during the 1600s and 1700s and in colours like bremen green and blue verditer.
Structure and Chemical Formula
The chemical formula of Copper Hydroxide is Cu(OH)2. Its molecule is an ionic compound that consists of the ions OH– and Cu2+.
The structure of copper hydroxide crystals was found from the method of X-ray crystallography. It was found that the central atom Cu is square pyramidal and the planar and axial lengths of Cu-O bonds are 1.96 Å and 2.36 Å respectively. Planar hydroxide ions either doubly or triply bridging other nearby copper atoms.
Properties of Copper Hydroxide
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
| Molar Mass | 97.561 g/mol |
| Appearance | Bluish green or pale greenish blue crystals |
| Density | 3.368 g/cm3 |
| Melting Point | 80C, decomposes to CuO |
| Solubility | insoluble in ethanol; negligible solubility in water; soluble in NH4OH |
| Standard Entropy of Formation (\(\Delta_f H^{\theta}_{298}\)) | -450 K J/mol |
| Odor | Characteristic copper odor |
Chemical Properties
- Reaction with ammonia
- The reaction of copper hydroxide and ammonia solution results in the formation of a deep blue colour solution of the complex tetraamminecopper complex ion([Cu(NH3)4]+2).
- In the presence of oxygen Cu(OH)2 catalyzes the oxidation of ammonia solutions, producing copper ammine nitrates, which are in the form Cu(NO2)2(NH3)x.
- It slightly dissolves in concentrated alkali and forms [Cu(OH)4]2-, as it is a mildly amorphous chemical.
- It reacts with sulfuric acid to form copper sulfate.
Cu(OH)2 + H2SO4 -> CuSO4 + 2H2O
- The bluish compound reacts with nitric acid to form copper nitrate.
Cu(OH)2 + 2HNO3 -> Cu(NO3)2 + 2H2O
Organic Reactions
Copper hydroxide is often used in organic synthesis by the addition of potassium hydroxide and a soluble copper(II) salt.
- It is used to produce aryl amines.
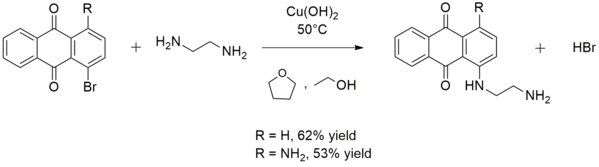
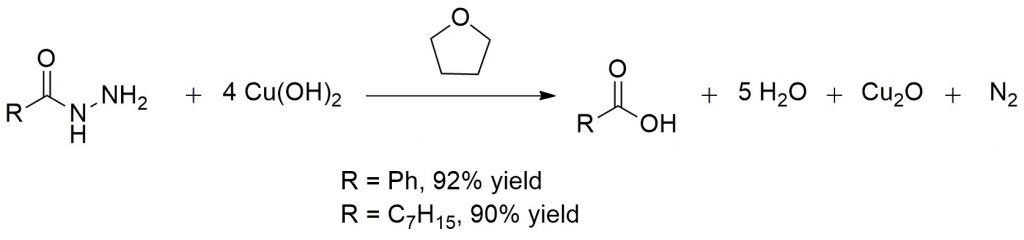
- It is used to convert acidic hydrazides to carboxylic acids. It is used to avoid any reaction with sensitive functional groups during the synthesis of carboxylic acids.
Production
- Copper hydroxide is produced from copper(II) sulfate by the reaction of its dilute solution and sodium hydroxide. However, the output of this method contains impurities like water and sodium hydroxide.
2NaOH(aq) + CuSO4(aq) -> Cu(OH)2(s) + Na2SO4(aq)
- It can also be produced by the electrolysis of water with the anode as copper.
- This compound is rarely found as a pure mineral it is mostly found combined with CO2 as basic copper(II) carbonate which is the mineral malachite. The mineral of Cu(OH)2 is spertinite.
2Cu(OH)2 + CO2 -> Cu2CO3(OH)2 (malachite) + H2O
Applications of Copper Hydroxide
- It is used in the production of rayon, as copper hydroxide in ammonia solution can dissolve cellulose. The solution of Cu(OH)2 in NH3 solution is known as Schweizer’s reagent.
- It is used to treat fish in the aquarium industry, as it can kill external parasites, flukes, marine ich, brook and marine velvet.
- It can be used as a replacement to the Bordeaux mixture, which is a fungicide and nematicide.
- Cu(OH)2 is also sometimes used as a colourant in the ceramic industry.
- It is used in a product which is used to control the root growth of a potted plant.
- Copper hydroxide and copper sulfate are used in pesticides and insecticides in the form of a mixture.
- It is also used as a wood preservative.

Hazards
- Excessive inhalation of its vapour can lead to cough and shortness of breath, among other things. If inhaled immediately go to fresh air. If the person is not breathing, provide artificial breathing.
- Its vapours are severe eye irritants and the direct contact of copper hydroxide with the eye can destroy the eye tissue. Wash with eyes open for 15 to 20 min until the chemical is washed.
- Prolonged skin contact can cause irritation and even allergies if repeatedly exposed to it. Wash for 15 to 20 min with soap until the chemical is washed off.
FAQs
It is slightly toxic upon ingestion. It does not cause death but, nausea, vomiting, salivation, gastric pain, diarrhea, capillary damage and liver and kidney damage are some of the many problems caused by its ingestion.
The name of the chemical Cu2CO3(OH)2 is copper carbonate hydroxide and it also exists as a mineral called Malachite.
It is bonded by an ionic bond between one Cu+2 and two OH– ions.
More on Inorganic Compounds
