The Aldol condensation reaction is a well-known organic reaction that involves the synthesis of β-hydroxy carbonyl compounds from aldehydes or ketones.
This reaction was first discovered by French chemist Charles-Adolphe Wurtz in 1872.
At the time, Wurtz was studying the reaction of acetaldehyde with various bases. Acetaldehyde on treating with a base undergoes a reaction. It produces a new compound having a sweet odor. Wurtz observed this and named this new compound “aldol” by combining the words “aldehyde” and “alcohol”.
Aldol reaction was also studied by other chemists in subsequent years and a variety of compounds were synthesized.
In 1884, the German chemist Heinrich Limpricht discovered the crossed aldol condensation, in which two different carbonyl compounds are used to form a β-hydroxyketone. This reaction has since become an essential tool in organic synthesis for the formation of new carbon-carbon bonds.
In the early 20th century, the mechanism of the aldol reaction was studied in detail by several prominent chemists, including Emil Fischer, Arthur Lapworth, and Paul Sabatier. They discovered that the reaction proceeds via an enolate intermediate, which is formed by the deprotonation of the α-carbon of the carbonyl compound.
Index
Explanation
The aldol condensation reaction is a type of organic reaction that typically involves the condensation of two carbonyl compounds, typically aldehydes or ketone, to form a β-hydroxy aldehyde or β-hydroxy ketone compound, followed by dehydration to give a conjugated enone.
The reaction occurs under basic conditions and proceeds through the formation of an enolate intermediate.
Aldol Condensation plays a vital role in organic synthesis, creating a path to form carbon-carbon bonds.
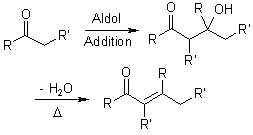
Mechanism:
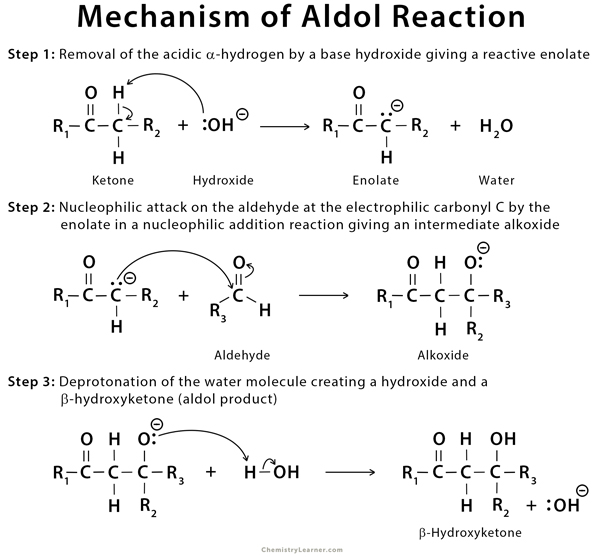
The reaction mechanism involves three steps:
- Formation of Carbanion: Under basic conditions, the α-carbon of the carbonyl compound is deprotonated to form a carbanion intermediate (enolate). This step is facilitated by the presence of a strong base, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH).
- Nucleophilic Addition/Attack: The carbanion intermediate then acts as a nucleophile, attacking the electrophilic carbon of the other carbonyl compound. This results in the formation of a carbon-carbon bond between the α-carbon of one carbonyl compound and the carbonyl carbon of the other. Thus, a alkoxide intermediate is formed.
- Formation of Aldol product: The intermediate then goes with the deprotonation of water and thus forms a hydroxide and β-hydroxy aldehyde or β-hydroxy ketone
This reaction can be classified into two types:
- Aldol condensation: The reaction involves the condensation of enol aka enolate and carbonyl compounds.
- Crossed aldol condensation: The reaction involves the condensation of two different carbonyl compounds to form a β-hydroxyketone.
Applications:
Important applications of this reaction are:
- The reaction is used in the synthesis of carbonyl compounds that are commonly used in the production of pharmaceuticals, etc.
- This plays a vital role in organic synthesis, creating a path to form carbon-carbon bonds which are used in the production of alcohol, isophorone, diacetone, etc.
- The process is also used as an intermediate in the process of perfume production, synthesis of pesticides etc.
Examples:
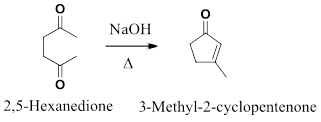
a) Intramolecular aldol condensation reaction
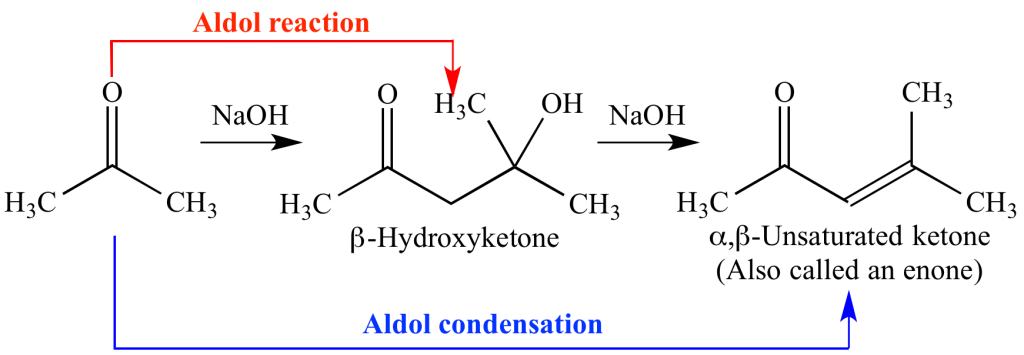
b) Crossed aldol condensation reaction
FAQ’S
Ethanol(C₂H₆O)
Yes, this can be catalyzed both by acids and bases.
No, the reaction can occur between two different carbonyl compounds, leading to the formation of a mixed aldol product.
No, it can also occur between other carbonyl compounds like esters and amides. Just that if it is not aldehydes and ketones, then the reaction is called something else.
More Organic Reaction
