Potassium Carbonate (K2CO3) is a white water-soluble salt. It is also known by the names carbonate of potash, dipotassium carbonate, sub-carbonate of potash, pearl ash, potash, salt of tartar, salt of wormwood. It is widely used in the manufacture of glass and soap.
Index
Discovery
The inorganic compound is the primary component of potash and refined pearl ash. Historically, pearl ash was created by baking potash in a kiln to separate it from impurities. Samuel Hopkins was awarded the first patent in 1780 for an improved method of making potash and pearl ash.
Structure & Formula
Potassium Carbonates’ formula is K2CO3. It is structurally represented as follows,
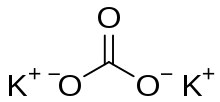
Properties of Potassium Carbonate
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
The physical properties of potassium carbonate are,
| Appearance | White, hygroscopic solid powder |
| Odor | Odorless |
| Solubility | Very soluble in water; insoluble in ethanol |
| Melting Point | 891℃ |
| Boiling Point | Decomposes |
Chemical Properties
The inorganic compound has the following chemical properties,
- Chemical formula – K2CO3
- Molar mass – 138.205 g/mol
- Density – 2.43 g/cm3
Production of Potassium Carbonate
The chemical compound is produced commercially by the reaction of potassium hydroxide with carbon dioxide.
2KOH + CO2 → K2CO3 + H2O
It can also be produced by treating potassium chloride with carbon dioxide in the presence of an organic amine to give potassium bicarbonate, which is then calcined.
2KHCO3 → K2CO3 + H2O + CO2
Applications of Potassium Carbonate
- The chemical compound was historically used for the production of soap, glass, and china products production.
- It is used as a fire suppressant in extinguishing deep-fat fryers.
- Also used as an animal feed ingredient to satisfy the potassium requirements of farmed animals such as broiler breeders.
- It is used as a buffering agent in the production of mead or wine.
- Also used in the alkalization of cocoa powder to produce Dutch process chocolate by balancing the pH of natural cocoa beans.
- It is used to soften hard water.
Hazards
Carbonate of potash, a.k.a, pearl ash causes skin, eye, and respiratory tract irritation when a person comes in contact with the compound.
FAQs
Potassium hydrogen carbonate has the chemical formula KHCO3.
The formula mass of potassium carbonate(K2CO3) is 138u.
It is used in food as its alkalinity controls acid flavours. It also stabilizes food colours, reduces bitter aftertaste, and regulates fat.
More on Inorganic Compounds
