Lithium bromide is a chemical compound of lithium and bromine. It has the formula LiBr. This compound can be produced by the action of hydrobromic acid on lithium hydroxide.
Index
Structure and Formula
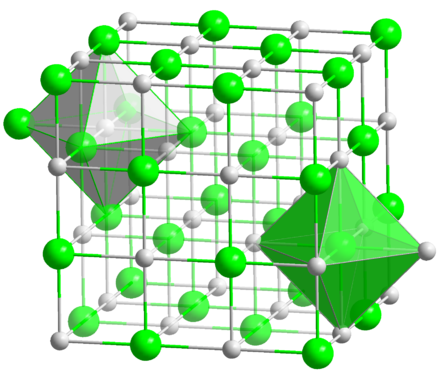
Lithium Bromide formula is LiBr and its structure is represented below.
Physical Properties
| Odor | Odorless |
| Appearance | White solid |
| Density | 3.464 g/cm3 |
| Melting Point | 550℃ |
| Boiling Point | 1,300℃ |
| Solubility | Soluble in water, methanol, ethanol, ether, acetone |
Chemical Properties
When the compound is made to react with chlorine, bromine is replaced by chlorine, thus giving lithium chloride and bromine. This is an example of a single displacement reaction.
Cl2 + 2LiBr → Br2 + 2LiCl
The compound, upon reaction with silver nitrate, gives lithium nitrate and silver bromide.
LiBr + AgNO3 → LiNO3 + AgBr
Applications
- The compound is used in air-conditioning systems as a desiccant.
- It is used as a salt in the absorption chilling along with water.
- Also useful as reagent in organic synthesis.
- It was used as a sedative in the early 1900s but later fell into disfavor due to newer sedatives, and other medical issues.
- It was used on treating bipolar disorder.
FAQs
It is used in the air-conditioning systems as a desiccant.
It may cause rash, nausea, diarrhea, drowsiness, and coma upon ingestion of small quantities. Ingestion of large quantities can cause kidney damage.
It is highly corrosive and readily attacks ferrous metals such as steel.
More on Inorganic Compounds
