E2 Reaction is an elimination reaction in which only one step is involved in eliminating two substituents from a molecule though it has a transitions state as well. E2 which is a bimolecular elimination reaction was suggested by British chemist Christopher Kelk Ingold in the 1920s.
Index
What is E2 Reaction?
Unlike E1 Reactions, with the inclusion of a solid base, E2 reactions eliminate two substituents, resulting in an alkene.
E2 usually exhibits second-order kinetics, and both the alkyl halide and the base appears in the rate equation.
Typically, E2 reactions are seen with secondary and tertiary alkyl halides, but with a primary halide, a hindered base is required.
E2 Reaction Mechanism
- The base attacks the neighbouring C-H bond and begins to remove the H at the same time as the alkene double bond starts to form and LG(Leaving Group – halide) group starts to leave.
- In this process, the hybridization of carbon must be reduced from sp3 to sp2 in order for the pi bond to be formed.
- In the rate-determining step, the C-H bond is weakened and a primary deuterium isotope effect far greater than 1 is thus observed.
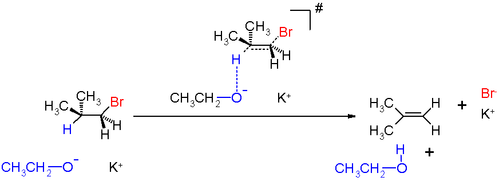
Characteristics of the Reaction Mechanism
- Mechanism Follows Second-Order kinetics.
- Takes place in One Step.
- Halides that are more substituted react more quickly.
Rate: R 3CX > R 2CHX > RCH 2 X X - Favoured by aprotic polar solvents.
- The two groups that leave should be on the same plane, which helps create a double bond with the reaction.
- The least substituted product results in a strictly impeded base, as it follows to Hoffman Rules.
- The key product is usually the most substituted alkene, as it follows Zaitsev law.
Factors Affecting the Rate of E2 Reaction
- The rate of the E2 reaction increases as the strength of the base increases.
- In general, heavy negatively charged bases such as OH are used out in E2 reaction.
- The rate of E2 reactions is increased by Polar aprotic solvents.
- The stronger the leaving group, the quicker the E2 reaction.
- The reaction rate follows the order, R-I > R-Br > R-Cl > R-F
Examples
Question 1. Which diastereomer of 1-bromo-4-t-butylcyclohexane cis or trans, undergoes elimination more rapidly when treated with sodium ethoxide? Explain your answer.
Answer. The ring is essentially locked up in the most stable conformation because of the inclusion of the bulky t-butyl group, with the bulky group being equatorial. Of the two isomers, the cis is the only one that fulfills the anticoplanar configuration for E2, where the leaving group and adjacent proton must be anti-equatorial and in the same plane.
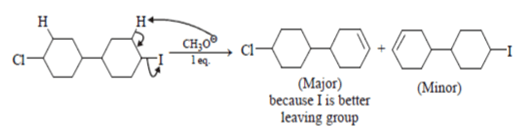
Question 2. Predict the product for the following elimination reaction.
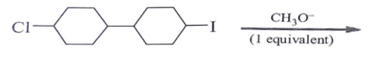
a)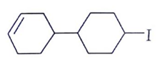
b) 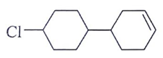
c) 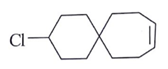
d) 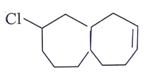
Answer: The product for above elimination reaction is (b).
Explanation: Iodine is a better leaving group than chlorine, so attack will be at iodine site.
FAQs
Generally, E2 reactions are carried out with strong, negatively charged bases such as OH and OR-. In the transition state, there is a partial breaking of the bond with the leaving group. So, the stronger the leaving group the quicker the E2 reaction.
Rearrangements do not occur in the E2 reaction.
E2 reactions are irreversible.
