Solvay Process is a process of producing soda from salt. It involves introducing carbon dioxide into the brine, which leads to the formation of sodium bicarbonate. This substance is subsequently heated through calcination to yield sodium carbonate (soda ash, Na2CO3).
Na2CO3 is a chemical compound commonly referred to as sodium carbonate, washing soda, or soda ash. It exists in different hydrated forms and is a colourless and scentless salt that easily dissolves in water, producing alkaline solutions.
Index
History
Ernest Solvay, a scientist from Belgium, created the Solvay Process in 1861.
It is often referred to as the Ammonia Soda Process or the Solvay Ammonia Process.
Explanation
This process is widely used in industry to produce sodium carbonate (Na2CO3), commonly known as soda ash.
This process utilizes easily accessible and affordable raw materials, such as:
1. Brine (a solution of sodium chloride)
2. Limestone (calcium carbonate)
3. Ammonia.
Brine, which is obtained from sources like seawater or underground water, provides the necessary sodium ions for the formation of sodium carbonate.
Limestone, on the other hand, serves as the source of carbonate ions needed in the synthesis of sodium carbonate, and can be obtained through mining.
While ammonia is a crucial component, it is synthesized using Haber’s process and can be quite expensive.
However, it is recycled helping to minimize costs and improve overall efficiency.
Steps involved
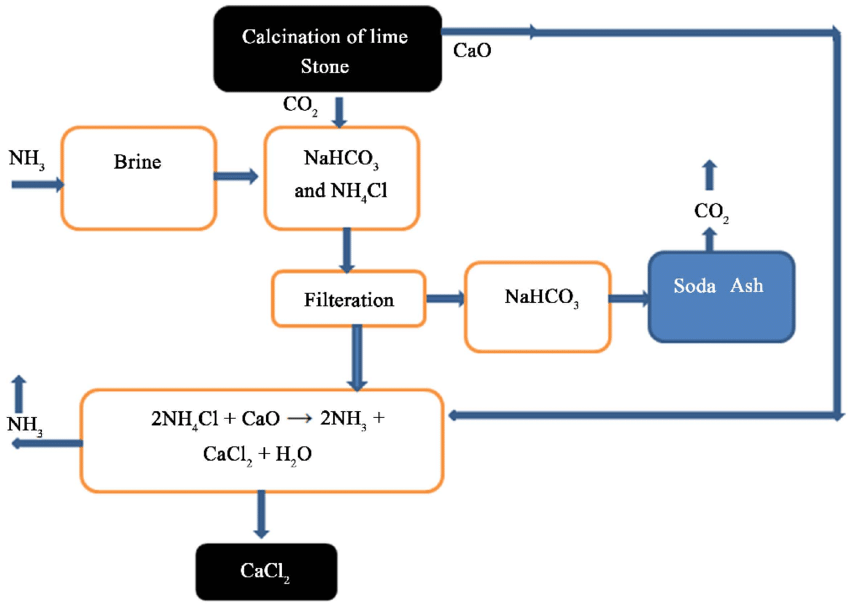
This process can be broken down into the following steps:
1. Brine is saturated with ammonia and CO2, and then cooled. This causes NaHCO3 to precipitate out of the solution, while NH4Cl is formed as a byproduct.
NaCl + NH3 + CO2 + H2O → NaHCO3(s) + NH4Cl
2. The precipitated NaHCO3 (sodium bicarbonate) is filtered, dried, and then heated. This leads to the formation of sodium carbonate (Na2CO3), carbon dioxide (CO2), and water vapor.
2NaHCO3 + Heat → Na2CO3 + H2O(g) + CO2(g)
3. Limestone is heated to produce CO2, which is used in step 1.
CaCO3 + Heat → CaO + CO2
Lime is added to water to produce calcium hydroxide (Ca(OH)2).
CaO + H2O → Ca(OH)2
The Ca(OH)2 is then reacted with the leftover solution from step 1, which contains NH4Cl. This results in the formation of CaCl2, NH3, and H2O.
Ca(OH)2(s) + 2NH4Cl(aq) → 2NH3(g) + CaCl2(aq) + 2H2O(l)
4. The NH3 produced in step 3 is recycled and used in step 1 for the production of NaHCO3.
Therefore, the only byproduct is Calcium chloride (CaCl2).
Environmental Issues Caused
However, it has various environmental issues linked to it, including thermal pollution, mining, and disposal of by-products, etc.
- Limestone, which is one of the raw materials for the Solvay Process, is obtained by mining. This leads to pollution and destroys natural habitats.
- The Solvay Process is exothermic, which means that an excess amount of heat is liberated into the atmosphere during the process. This causes a rise in temperature, leading to thermal pollution. The increased temperature in the waterways can negatively impact aquatic life, including fish eggs.
- The main by-product of the Solvay Process is calcium chloride, which is produced in large quantities. Disposing of this by-product is difficult without affecting the ecosystem, as the high chloride ion concentration can negatively affect vegetation in the area.
- A small amount of ammonia is also released during the Solvay Process, which can cause respiratory problems if inhaled.
Applications
- Soda ash, which is also known as sodium carbonate (Na2CO3), is commonly produced using the Solvay process.
- Sodium carbonate which is produced using solvay process has various applications in several industries including:
- Glass manufacturing
- Cleaning formulations
- Chemical production, etc.
FAQ’S
Sodium Carbonate (Na2CO3) also known as Soda Ash.
Potassium carbonate (K2CO3) cannot be obtained through the Solvay process. The reason is that unlike sodium bicarbonate potassium bicarbonate (KHCO3) dissolves relatively easily in water and does not undergo precipitation.
Calcium chloride (CaCl2)
Ammonia-soda process
Because it uses inexpensive and plentiful raw material to prepare useful chemicals like Na2CO3.
Related Topics
