Ammonium phosphate is an inorganic compound with the chemical formula of (NH4)3PO4. This compound is the ammonium salt of the ortho-phosphoric acid.
Index
Structure
The structure of the chemical compound is shown below.
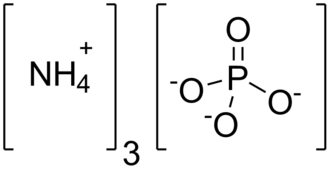
The IUPAC name of this compound is also ammonium phosphate. This compound is highly unstable.
Ammonium Phosphate Preparation
This chemical compouund is manufactured commercially by mixing phosphoric acid with ammonia. Commercial-grade ammonia is usually obtained in crystalline powder form.
Properties of Ammonium Phosphate
Now let us discuss the properties of this compound where we will look at its physical and chemical properties.
Physical Properties
| Molecular Formula | (NH4)3PO4 |
| Molecular Weight(amu) | 149.09 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass of the compound | 149.0565 |
| Topological Polar Surface Area | 89.2 Å2 |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 36.8 |
| Isotope Atom Count | 0 |
| Is it Canonicalized? | Yes |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
| Physical Appearance | White Tetrahedral Crystals |
| Density (g/cm3) | 1.619 |
| Melting Point (℃) | 130 |
| Boiling Point (℃) | 155 |
| Solubility in water(g/100mL) | 58.0 |
Chemical Properties
The compound readily undergoes decomposition to give phosphoric acid and ammonia.
(NH4)3PO4 → 3NH3 + H3PO4
It readily reacts with lead nitrate(Pb(NO3)4) to give ammonium nitrate(NH4NO3) and lead phosphate(Pb3(PO4)4).
4(NH4)3PO4 + 3Pb(NO3)4 → Pb3(PO4)4 + 12NH4NO3
Hazards
Exposure to this compound may cause skin and eye irritation.
Applications
- The compound holds a generic name for a variety of fertilizer materials.
- It finds an important application as a fertilizer.
- The inorganic compound is used as a component of intumescent paints and mastics.
- It is also used as a plant revitalizer.
FAQs
The chemical formula of the compound is (NH4)3PO4.
The molar mass of the compound is 149.09amu.
It is used as a fertilizer, and also as a flame retardant in thermoplastic compositions.
More on Inorganic Compounds
