Ammonium acetate is a chemical compound that is also known as the Spirit of Mindererus in an aqueous solution. It is derived from the reaction of ammonia and acetic acid.
Index
Structure & Formula
You can see the chemical structure of the chemical below.
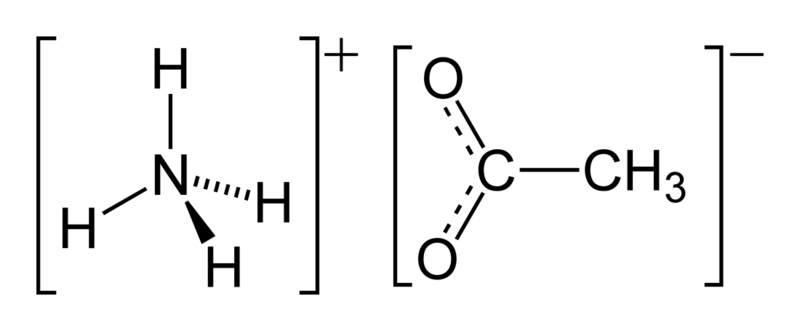
This compound has the chemical formula of NH4CH3CO2. It has the IUPAC name of Ammonium ethanoate.
Ammonium Acetate Preparation
The compound is prepared by saturating glacial acetic acid with ammonia. It is also produced by the neutralization of acetic acid with ammonium carbonate.
Properties of Ammonium Acetate
Let us discuss some of the important properties of this chemical compound.
| Molecular Weight(amu) | 77.08 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass of the compound | 77.0476 |
| Topological Polar Surface Area | 41.1 Å2 |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Is it Canonicalized? | Yes |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| Physical Appearance | White Solid Crystals, deliquescent |
| Odor | Slightly acetic |
| Density (g/cm3) | 1.17 |
| Melting Point (℃) | 113 |
| Boiling Point (℃) | – |
| Solubility | Soluble in alcohol, acetone, liquid Ammonia, SO2 |
Hazards
Inhalation of ammonium acetate dust may cause nose and mouth irritation. Swallowing of the compound may cause stomach irritation. Eye or skin contact with it may cause rashes.
Applications
- The compound finds its applications in explosives, foam rubbers, vinyl plastics, etc.
- It is also used to preserve meats and dyes.
- It finds application as a biodegradable de-icing agent.
- The compound is used as a catalyst in the Knoevenagel reaction and also as a source of ammonia in the Borch reaction.
- It is used as a reagent in agricultural chemistry for the determination of soil CEC(cation exchange capacity).
- It is also used as a food additive, as an acidity regulator.
- Ammonium Acetate is the main precursor to acetamide.
FAQs
It should be kept cool and tightly stored.
The chemical formula of this chemical compound is NH4CH3CO2.
It finds major application in preserving meats and dyes, in explosives, foam rubber, etc.
More on Inorganic Compounds
