Lead nitrate is an inorganic compound. It is also known as Plumbous Nitrate. It has the chemical formula Pb(NO3)2.
The inorganic compound was first identified by alchemist Andreas Libavius who called the substance as Plumbus Dule meaning sweet lead because of its taste in 1597.
Index
Structure & Formula
The chemical formula of Lead nitrate is Pb(NO3)2 and its structure is represented as below. The colour of the compound is white or colourless.
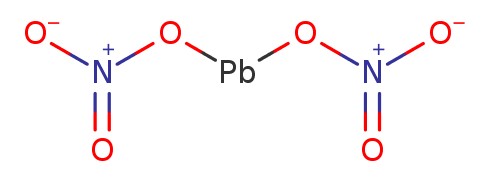
Preparation Method
Let’s look at the preparation method of this chemical compound.
Lab Preparation Method
This compound is produced in the lab by the reaction of Lead oxide with concentrated Nitric acid.
PbO + 2 HNO3(conc) -> Pb(NO3)2 + H2O
Commercial Preparation Method
The compound is commercially obtained as a byproduct in the manufacture of Lead.
Properties of Lead Nitrate
Now, let’s move on to the discussion of the physical and chemical properties of the compound.
Physical Properties
| Odour | Odourless |
| Colour | White or no colour |
| Melting Point | 470°C |
| Solubility | Highly soluble in water, Alkali and Ammonia |
| Boiling Point | Decomposes |
| Appearance | White crystalline solid |
Chemical Properties
Lead Nitrate with Potassium Iodide: When the compound reacts with potassium iodide the iodide ion replaces Nitrate ion and thus resulting in an Double displacement reaction.
Pb(NO3) 2 (aq) + KI → PbI2(s) + KNO3(aq)
Reaction with O2: When the compound is heated, it decomposes into lead oxide(PbO), Nitrogen dioxide(NO2) and oxygen gas. This reaction is also called the Thermal decomposition reaction.
Pb(NO3) 2 + O2 → PbO + NO2 +O2
Applications
- The chemical compound is used in making match sticks and special explosives.
- It is used in metallurgical process in leaching of Gold.
- It is used in dye and photographic industry and in process engraving
- The compound has been employed as heat stabilizer in nylon and polyester as a photo thermo-graphic paper coating.
Hazards
- The compound is probable carcinogenic in humans
- It causes cancer to lungs and kidneys
FAQs
It doesn’t react with water because it is highly soluble in water
It tastes like sweet lead.
Lead nitrate does not give Brown ring test because Lead reacts with freshly prepared Ferrous sulphate forming lead sulphate(white ppt) that obscures the brown ring
More on Inorganic Compounds
