The term “Amphoteric” is derived from the Greek word amphoteroi (ἀμφότεροι), which means “both”. Amphoteric oxides are oxides of elements (usually metals) that show amphoteric behaviour.
Amphoterism or amphoteric behaviour is the property of a compound to act as both an acid and as a base. Depending upon reactants and other reaction conditions, the compound behaves either like acid or as a base.
Example of amphoteric oxides: Aluminium Oxide Al2O3 is an amphoteric oxide because it can neutralise both HCl and NaOH.
Other elements which form amphoteric oxides are gallium, indium, scandium, titanium, zirconium, vanadium, chromium, tin, iron, cobalt, copper, zinc, lead, silver, gold, germanium, antimony, bismuth, beryllium and tellurium.
Index
Synthesis
Since, amphoteric oxides are oxides of certain elements, therefore they are a direct result of burning those elements in air. For example Zinc(II) oxide is produced as a result of direct combustion of Zn in plenty of supply of air:
2Zn(s) + O2(g) -> 2ZnO(s)
Similarly,
4Al(s) + 3O2(g) -> 2Al2O3(s)
Lead (II) oxide is produced by heating Lead (II) Nitrate:
2Pb(NO3)2 -> \(\Delta\) 2PbO(s) + 4NO2 + O2(g)
Tin(II) oxide also known as Stannous Oxide could be produced by heating Tin(II) Oxide in an inert environment. Tin(II) Oxide is formed when Tin salts react with alkali such as Sodium Hydroxide.
SnCl2 + 2NaOH -> Sn(OH)2 + 2NaCl
Sn(OH)2 -> \(\Delta\) SnO + H2O
Amphoteric Oxides in Periodic Table
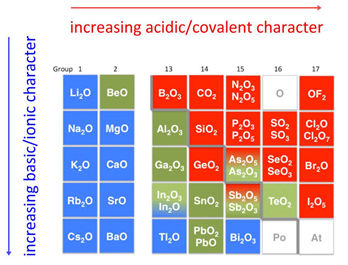
- In a given period of the periodic table, the oxides progress from basic to amphoteric and finally to acidic. For example consider 3rd period elements: Na2O and MgO are basic , Al2O3 is amphoteric, SiO2 is weakly acidic, P2O5 , P2O3, SO2, SO3 , Cl2O and Cl2O7 are acidic.
- Down the groups, basicity increases with increasing atomic number.
Consider group 15 oxides:
NO2 and P2O3 are acidic, As2O3 and Sb2O3 are amphoteric , Bi2O3 is basic. - Acidity also increases with an increase in the oxidation number of the same elemental oxide. Example: Acidity series for manganese oxides is
Mn2O7 > Mn2O3 > MnO
Similarly, for Arsenic and Antimony,
As2O5 > As2O3
and
Sb2O5 > Sb2O3
Physical Properties of Amphoteric Oxides
Amphoteric oxides usually have very high melting and boiling points. They have giant covalent structures which require a lot of energy to break.
Amphoteric oxides are insoluble in water.
Chemical Properties of Amphoteric Oxides
Amphoteric oxides react with both acids and bases.
Basic Nature
The basic nature of amphoteric oxides is made clear by their reaction with acids. Amphoteric oxides react with common inorganic acids to form metal salt and water.
ZnO(s) + 2HNO3(aq) -> Zn(NO3)2(aq) + H2O(l)
Al2O3(s) + 6HCl(aq) -> 2AlCl3(aq) + 3H2O(l)
SnO(s) + 2HCl(aq) -> SnCl2(aq) + H2O(l)
PbO(s) + 2HNO3(aq) -> Pb(NO3)2(aq) + H2O(l)
As2O3(s) + 6HCl(aq) -> 2AsCl(aq) + 3H2O(l)
Acidic Nature
The acidic nature of amphoteric oxides is made clear by their reaction with bases. Amphoteric oxides react with common inorganic bases to form complex metal salts and water.
ZnO(s) + 2NaOH(aq) -> Na2ZnO2(aq) + H2O(l)
Note that Na2ZnO2 is compounded with water in reality.
So the more accurate equation is:
ZnO(s) + 2NaOH(aq) + H2O(l) -> Na2[Zn(OH)4](aq)
where Na2[Zn(OH)4] is hydrated sodium Zincate and the anion is [Zn(OH)4]+2 .
Al2O3(s) + 2NaOH(aq) -> 2NaAlO2(aq) + H2O(l)
or
Al2O3(s) + 2NaOH(aq) + 3H2O(l) -> 2Na[Al(OH)4](aq)
where Na[Al(OH)4] is hydrated Sodium Aluminate and [Al(OH)4]+1 is the anion.
PbO(s) + 2NaOH(aq) + H2O(l) -> Na2[Pb(OH)4](aq)
SnO(s) + 4NaOH(aq) + H2O(l) <=> Na4[Sn(OH)6](aq)
Identification of Amphoteric Oxides
An amphoteric oxide can neutralise both an acid and a base. So, in order to identify if a compound is amphoteric, its reaction with an acid such as HCl and reaction with a base such as NaOH need to be observed.
After confirming that the given substance is amphoteric, we need to find out if the given substance is an oxide or not. Tests for other anions like Sulphate, Nitrate, Nitrite, Chloride etc., are done. If all tests show negative results, the given compound is an oxide.
Uses and Applications
Common uses of some amphoteric oxides are:
- Lead(II) Oxide: Adding PbO to glass increases its electrical resistance, refractive index and ability to absorb X-Rays. At the same time, it decreases the viscosity of glass. Hence PbO is essential for the glassware industry.
PbO is also used in ceramic industries to make ceramic articles that are magnetically and electrically inert. - Zinc Oxide: Zinc Oxide is used extensively in the cement and cosmetic industries. It is also used to make dry cells and other types of permanent cells.
- Aluminium Oxide: Aluminium oxide ores such as bauxite are present in the Earth’s crust. First, these ores are concentrated to form pure Aluminium Oxide. This Aluminium Oxide is then used in the Hall-Héroult process to obtain pure aluminium metal.
FAQs
Amphoteric oxides are oxides of elements (usually metals) that show amphoteric behaviour. Amphoterism or amphoteric behaviour is the property of a compound to act as both an acid and as a base. Depending upon reactants and other reaction conditions, the compound behaves either like acid or as a base.
An amphoteric oxide can neutralise both an acid and a base. So to identify if a compound is amphoteric, its reaction with an acid such as HCl and reaction with a base such as NaOH need to be observed.
After confirming that the given substance is amphoteric, we need to find out if the given substance is an oxide or not. Tests for other anions like Sulphate, Nitrate, Nitrite, Chloride etc., are done. If all tests show negative results, the given compound is an oxide.
Yes, water is an amphoteric compound.
H2O <=> H3O+ + OH–
Acidic behaviour:
H2O(acid) + NH3(base) -> NH4+ + OH–
Basic behaviour:
H2O(base) + HCl(Acid) -> Cl– + H3O+
