Barium acetate is a salt of barium(II) and acetic acid. It is toxic to humans, but has use in chemistry and manufacturing.
Index
Structure & Formula
It has the chemical formula Ba(C2H3O2)2. This compound is also known as acetic acid and barium salt. The 2D structure of the compound is displayed below.
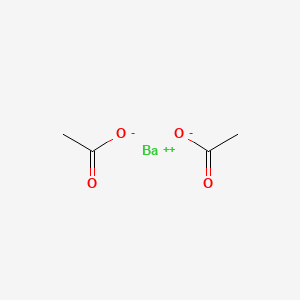
The compound has the IUPAC name as barium(2+) diacetate.
Preparation
Barium acetate is produced by the reaction of acetic acid with barium carbonate,
BaCO3 + 2CH3COOH → (CH3COO)2Ba + CO2 + H2O
This reaction takes place in solution and the desired product crystallizes out.
Barium sulfide can also be used instead of barium carbonate to obtain crystallized barium acetate,
BaS + 2CH3COOH → (CH3COO)2Ba + H2S
Chemical & Physical Properties
| Molecular Weight (amu) | 255.41 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass of the compound | 255.931856 |
| Topological Polar Surface Area | 80.3 Å2 |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Is it Canonicaliized? | Yes |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| Physical Appearance | White Crystals or White Powder |
| Odor | Odorless |
| Density (g/cm3) | 2.47 |
| Melting Point (℃) | 450 |
| Solubility in water(g/100ml) | 59 |
Reactions
Barium acetate, upon heating, decomposes to carbonate. It reacts with sulfuric acid to give sulfate, hydrochloric acid to give chloride, and nitric acid to give nitrate.
Hazards
The chemical compound is harmful if swallowed or inhaled.
Applications
- The chemical compound finds application as a mordant for printing textile fabrics.
- It is also used for drying paints and varnishes.
- The compound is also used in lubricating oils.
- Barium acetate, in chemistry, is used as a catalyst in organic synthesis.
FAQs
The compound is prepared by the reaction of acetic acid with barium carbonate.
BaCO3 + 2CH3COOH → (CH3COO)2Ba + CO2 + H2O
It has a molar mass of 255.415 g mol-1.
The compound has the chemical formula Ba(C2H3O2)2.
More on Inorganic Compounds
