Zinc carbonate (ZnCO3) is a naturally occurring mineral and is also commonly called as Smithsonite or Calamine or Zinc spar.
Index
Structure and Formula
The chemical formula of Zinc carbonate is ZnCO3 and it has the following structure,
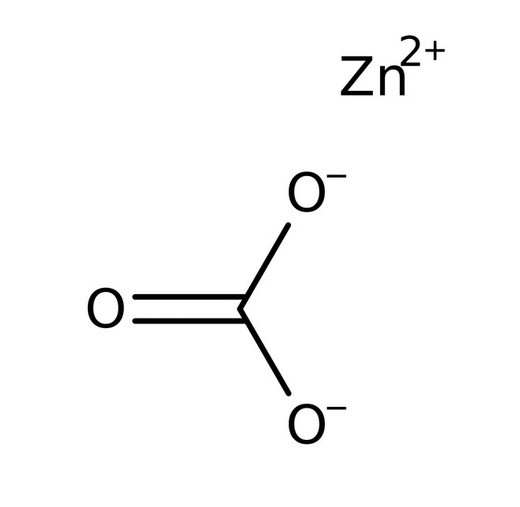
Properties
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
The compound has the following physical properties,
| Appearance | White crystalline (In pure form but generally coloured by Iron, Manganese etc impurities ) |
| Odor | Odorless |
| Zinc Carbonate Solubility | Insoluble in water, soluble in dilute acids and alkalis |
| Melting point | 300 °C (572 °F; 573 K) (decomposes) |
| Boiling point | Decomposes |
Chemical Properties
It has the following chemical properties,
- Chemical formula – ZnCO3
- Molar Mass – 125.38 g/mol
- Density – 3.5 g/cm3
- ZNCO3 reacts with acids like hydrochloric acid to form zinc chloride and release carbon dioxide.
ZNCO3 + HCl → ZnCl2 + CO2
Applications of Zinc Carbonate
- ZnCO3 is used in ointments.
- It is used in dusting upon inflamed surfaces as an astringent and absorbent.
- It is also used as a food additive.
- Also used as a vulcanization activator.
Hazards of Zinc Carbonate
- Inhalation of zinc dust or fumes leads to dry throat, cough, and chest discomfort. It may also cause nausea, fever, and sweating.
- It has been found to be highly toxic to aquatic life leading to long-lasting harmful effects.
FAQs
What is the chemical formula of zinc carbonate?
Its chemical formula is ZnCO3.
Is zinc carbonate good for the skin?
ZnCO3 is applied liberally to inflamed skin in the form of powder, calamine lotion, etc.
What does zinc carbonate do in shampoo?
ZnCO3 helps fight flakes by moisturizing the scalp, and when paired with ZPT, it helps keep the dandruff-fighting ingredient in its most effective form.
More Inorganic Compounds
