Magnesium phosphate is an important mineral found in bones, in the seeds of many plants, and in a number of minerals. It has the formula Mg3(PO4)2.
Index
Structure and Formula
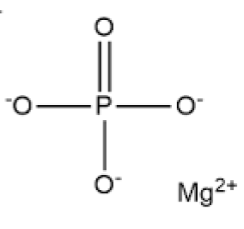
The chemical formula of this compound is given as Mg3(PO4)2 and its structure is shown below.
Physical Properties
| Odor | No odor |
| Appearance | White crystalline powder |
| pH | 6 – 9.5 |
| Solubility | Soluble in salt solution |
| Melting Point | 1,184℃ |
| Boiling Point | – |
Chemical Properties
Magnesium phosphate when treated with water gives as product, phosphoric acid, and magnesium hydroxide.
Mg3(PO4)2 + H2O → H3PO4 + Mg(OH)2
The chemical coumpound forms phosphoric acid and magnesium chloride, upon reaction with hydrochloric acid.
Mg3(PO4)2 + HCl → MgCl2 + H3PO4
Applications
- The mineral finds application in the prevention of vitamin E deficiency.
- It is used as a nutrient, pH control agent, and stabilizer.
- It is used in the minimal supplement.
FAQs
What is the formula for magnesium phosphate?
The chemical formula of this compound is given as Mg3(PO4)2.
What is the melting point of magnesium phosphate?
The melting point is 1,184℃.
What does magnesium phosphate smell and taste like?
It does not have an odor. It is also tasteless.
More on Inorganic Compounds
