The Clemmensen reduction is a chemical reaction that allows the reduction of ketones to alkanes using zinc amalgam and concentrated hydrochloric acid.
This reaction is named after Danish chemist, Erik Christian Clemmensen.
Index
The Reaction
The reduction reaction is as follow:
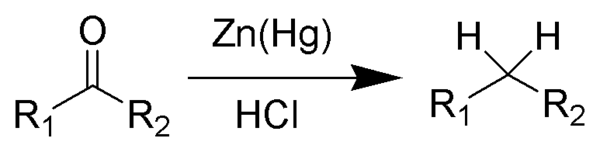
Clemmensen Reduction Mechanism
The reduction reaction allows the deoxygenation of aldehydes or ketones to produce a hydrocarbon.
Scientists have tried to understand the mechanism of the reduction reaction but were able to only put forward two proposals:
Carbanionic Mechanism
In this mechanism, the Zinc catalyst directly attacks the protonated carbon.
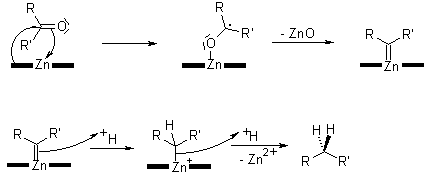
Carbenoid Mechanism
The reduction of the ketone takes place at the surface of the zinc catalyst. The alcohols are not postulated as intermediates, as the subjection of the corresponding alcohols to the same reaction conditions does not lead to alkanes.
The above-illustrated reduction reaction is effective in reducing aryl-alkyl ketones, which are the products of Friedel-Crafts Acylation. Primary alkylation of arenes can be achieved by following the two-step sequence of Friedel-Crafts acylation and then, Clemmensen reduction.
In this reaction, the substrate must be tolerant of the strongly acidic conditions of the reduction, i.e., 37% HCL. There are several alternatives for this reaction, such as the Wolf Kishner reduction for acid-sensitive substrates.
The mechanism of the reaction remains obscure, in spite of its antiquity. Mechanistic studies have been difficult to conduct due to the heterogeneous nature of the reaction.
Applications of Clemmensen Reduction
- The reduction mechanism is widely used to convert the carbonyl group to the methyl group.
- It is also used in the preparation of polycyclic aromatics and aromatics containing unbranched side hydrocarbon chains.
- It is widely used to transform acyl benzene, obtained from Friedel-Crafts acylation reaction to alkylbenzene.
- The reduction reaction, in the presence of zinc amalgam and concentrated hydrochloric acid, converts benzaldehyde to toluene.
FAQs
The reaction is a chemical reaction that reduces ketones to alkanes with the help of Zinc amalgam and concentrated HCL.
No, it does not reduce alcohol, as there is no formation of alcohol during the reaction.
Zinc amalgam catalyst is used in this reduction.
More Organic Reactions
