Newlands’ Law of Octaves is an early attempt to classify elements based on periodic properties. It set the stage for later, more complete methods like Mendeleev’s and the Modern Periodic Table.
Index
History
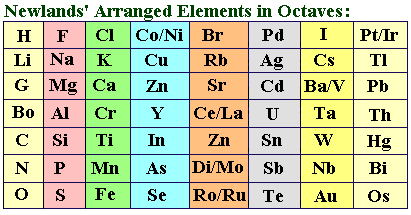
Newlands’ Law of Octaves was proposed in 1865 by John Newlands, a British chemist. He arranged the known elements in increasing order of atomic weight, and suggested that after every eight elements shared properties were noticed. This he compared to a musical term called the “octave” for 8 notes.
His work was an improvement over previous work such as that of Dobereiner, who made a smaller attempt called Dobereiner Triads.
However, Newlands Law of Octaves was not taken seriously by the scientific community at that time.
Features of Newlands Octaves
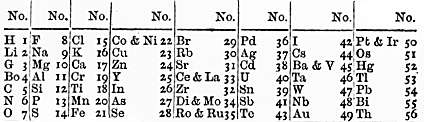
Here are some major features of Newlands’ Law of Octaves
- Newlands Octaves arranged the known 62 elements then into roughly 8 groups of 8 each.
- The elements were in increasing order of atomic weight.
- Every element shared properties with the 8th element after it.
- Sometimes two elements were made to occupy one place, to reflect their properties.
This was one of the largest early attempts to arrange all known elements in a specific order.
Limitations of Newlands Law of Octaves
Newlands’ Octaves had some limitations which we discuss below.
- While the groups had similar features, there were also some dissimilar elements in same group. For example, metals like platinum, cobalt and nickel, were in the same group as halogens like chlorine and bromine.
- In some slots, two elements had to share the same place. For example, cobalt and nickel, or barium and vanadium. There was no clear explanation for this.
- The law strictly worked well only upto Calcium, for heavier elements the relationship began to break down.
- There were no spaces left for future elements that were discovered. This meant that future elements disrupted the pattern.
FAQs
Newlands Law of Octaves was an early attempt at classifying elements in a periodic manner. It states that, “when elements are arranged in order of increasing atomic mass, every 8th element has similar properties to the first.”
It divided the known elements at that time into around 8 groups of 8 each.
The limitations of Newlands Law of Octaves are as follows:
1. It left no space for new elements to be discovered.
2. It grouped some dissimilar elements together, e.g. halogens with metals.
3. It worked well only upto Calcium.
4. Sometimes two elements had to take up one place, with no explanation.
Some early attempts at periodic classification of elements were:
1. Dobereiner’s Triads
2. Newlands’ Octaves
3. Lothar Meyer’s Curve
4. Dmitri Mendeleev’s Periodic Table
Atomic weight of the elements and its relation to physical and chemical properties was commonly used in all these methods.
