SN1 and SN2 reactions are two kinds of organic reactions. Specifically, they are “Nucleophilic Substitution Reactions”. We are going to discuss the terminology involved here and find out the difference between SN1 and SN2 reactions.
Index
Terminology
Nucleophilic: This means that the reagent is drawn to positive charge. The reagent can be neutral or negatively charged, but it must have an excess of electrons.
Substitution: The reagent comes and replaces a functional group in the organic compound. In the case of nucleophilic substitution, the incoming reagent has excess electrons. It attacks a functional group with lesser electron density.
SN1 and SN2: The Difference
Both SN1 and SN2 reactions are nucleophilic substitution reactions. The difference lies in number of molecules involved in the key reaction steps.
SN1 is unimolecular. This means that the rate-defining step involves only one molecule.
SN2 is bimolecular. This means that the rate-defining step involves two molecules.
These major differences give rise to various other differences between SN1 and SN2 reactions, which are tabulated below.
| Feature | SN1 reaction | SN2 reaction |
| Molecularity of Reaction | SN1 reaction is a unimolecular reaction. | SN2 reaction is a bimolecular reaction. |
| Rate Law | SN1 reaction rate law is a first-order rate law. | SN2 reaction rate law is a second-order rate law. |
| Number of Steps | It is a two-step process. | It is a one-step process. |
| Rate depends on | The reaction rate depends only on the concentration of organic reactant (substrate). | The reaction rate depends on both the concentration of the reactant (substrate) and the reagent (nucleophile). |
| Mechanism | The functional group to be replaced first exits, forming carbocation. Then, reagent attacks the carbocation. | The reagent attacks the substrate at the same time as the replaced group is leaving, forming a transition state. |
| Optical Properties | If the substrate is optically active, the end product is optically inactive. It will contain equal amounts of both optical isomers. | The product will have opposite chirality when compared to substrate. This is called optical inversion. |
| Reaction Intermediates | There is a carbocation formed as a reaction intermediate. | There is a high energy transition state formed as a reaction intermediate. |
SN1 and SN2: Differences in Mechanisms
Here, we have the mechanisms of SN1 and SN2 reactions, in diagram form. We can see the difference between SN1 and SN2 reaction mechanisms.
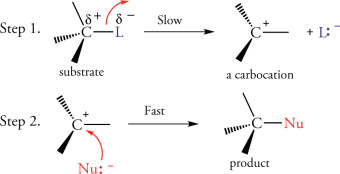
We can see the two-step nature of the SN1 reaction above.
On the other hand, the one-step nature of the SN2 reaction is given below.
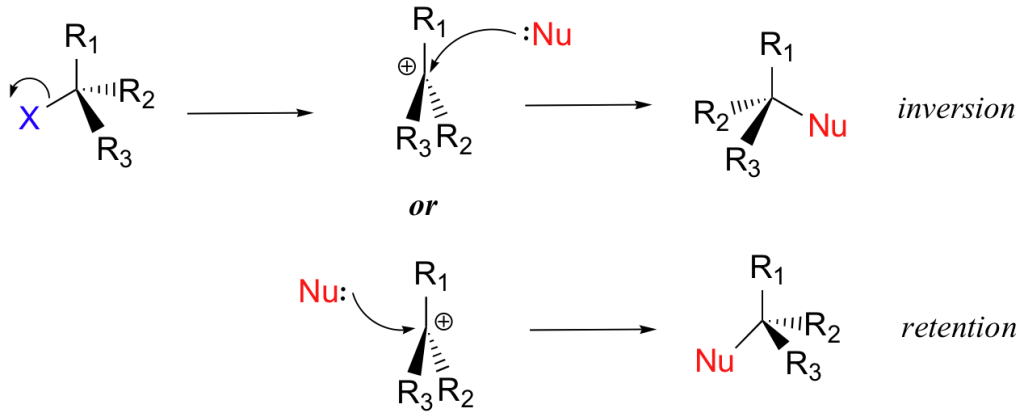
Know more on reaction mechanisms.
FAQs
Some examples of nucleophilic reagents are:
– Halogen ions (Cl–, Br–, I–)
– Ammonia (NH3)
– Cyano group (CN)
– Hydroxyl group (OH)
The reaction intermediate in SN1 reaction is a carbocation. It is planar and can be attacked from both sides with equal probability. Thus, both kinds of optical isomers are produced in equal concentrations. The mixture as a whole is optically inactive. This process is called racemization.
A stereospecific reaction means that the end-product varies based on which optical isomer we use in the reactant. As we know, SN2 reaction involves optical inversion of the reactant. As such, an R-isomer in reactant gives rise to S-isomer in the product.
Similarly, an S-isomer in reactant gives an R-isomer in the product. Thus, the SN2 reaction is said to be stereospecific.
The major differences between SN1 and SN2 reactions are as follows:
1. Molecularity: SN1 is unimolecular, while SN2 is bimolecular.
2. Mechanism: SN1 proceeds via carbocation intermediate, SN2 involves a transition state.
3. Optical property: SN1 reaction leads to racemization, SN2 reaction leads to optical inversion.
4. Rate of reaction: SN1 follows first order kinematics, while SN2 follows second-order kinematics.
5. Number of steps: SN1 reaction involves two steps, while SN2 involves one step.
