E1 Reaction is an elimination reaction in which two-steps: ionization and deprotonation are involved in eliminating two substituents from a molecule.
Index
What is E1 Reaction?
Unimolecular Elimination (E1) is a reaction that results in the formation of a double bond by removing an HX substituent. It is identical in different ways to the unimolecular nucleophilic substitution reaction (SN1 Reaction). The formation of a carbocation intermediate is one of the similarity.
The dissociation of the leaving group to form a carbocation is also the only rate-determining (slow) process here, hence the name unimolecular elimination.
Many times, both SN1 and E1 reactions will occur simultaneously from a single reaction to form different products. By thermodynamic regulation, however, one may be preferred over another.
E1 Reaction Mechanism
E1 indicates an elimination, unimolecular reaction, where rate = k [R-LG].
Where,
LG = leaving group
R = carbon chain involved in reaction
This suggests that the mechanism’s rate-determining step relies on the decomposition of a single molecular species.
Overall, with the following two key stages, this pathway is a multi-step Process.
Step 1: Loss of the leaving group (LG), to generate a intermediate carbocation.
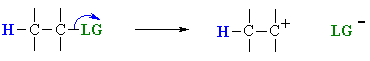
Step 2: Loss of a proton, H+, from the carbocation to form the π-bond.
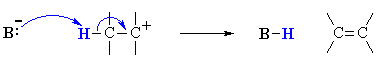
Characteristics of Reaction Mechanism
- Follows First order kinetics.
- Two steps Mechanism.
- More substituted halides react faster.
Rate: R 3CX > R 2CHX > RCH 2 X - Weaker bases such as H2O and ROH are favoured.
- A better leaving the group leads to faster reaction rates.
- A polar protic solvents, which can stabilize the ionic intermediates is favoured.
Selectivity of E1 Reactions
E1 reactions mostly favour the more stable alkene as the major product.
Favourable conditions for E1 reactions are:
- good leaving groups.
- stable carbocations.
- weak bases.
- E1 reactions are also regioselective (Any process that favours bond formation at a particular atom over other possible atoms.) and follow Zaitsev’s rule.
Examples
Question 1. Explain why presence of weak base prefers E1 over E2 ?
Answer. E1 reactions are a Unimolecular Elimination Mechanism, which means the rate-determining step is the dissociation of the leaving group to form a carbocation. Since E2 is bimolecular and the nucleophilic attack is a part of the rate-determining step, a weak base/nucleophile disfavors it(E2) and ultimately allows E1 to dominate.
Question 2. What is the major product for following reaction?
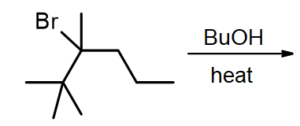
Answer. The major product formed is:
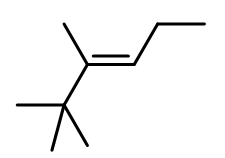
Question 3. Which type of elimination took place here?
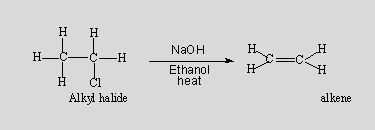
Answer. This is an E1 reaction.
Question 4. Which of the following statements is correct for alkyl halide?
- Alkyl halide will always show SN1 mechanism.
- As branching at carbon increases, E1 mechanism is favoured as compared to SN1 mechanism.
- In unimolecular reaction, increasing the temperature does not favour E1 mechanism.
- In most unimolecular reactions of alkyl halide, E1 reaction is favoured over SN1 reaction.
Answer. Statement 2 is Correct.
Explanation: In most unimolecular reactions of alkyl halide SN1 reaction is favoured over E1 reaction. E1 mechanism is favoured as compared to SN1 mechanism by branching at carbon increases, as more steric hindrance at the attacking site will lead to E1 mechanism.
FAQs
The difference between substitution and elimination reactions is that in substitution reactions, replacing of atoms takes place, while the replacement is not observed in elimination reactions.
An E1 reaction involves the deprotonation near the carbocation of a hydrogen (usually one carbon apart, or the beta position) leading to the forming of an alkene component.
Yes, E1 reactions are regioselective.
