Dimethylglyoxime (C4H8N2O2) is basically a white powder abbreviated as dmgH2 for neutral form and dmgH for anionic form, where H stands for hydrogen.
Index
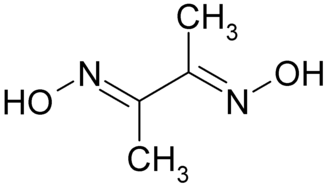
Structure & Formula
The chemical formula of Dimethylgloxime is C4H8N2O2 (CH3C(NOH)C(NOH)CH3) and the structure for dimethylglyoxime is shown below.
Properties of Dimethylglyoxime
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
| Odor | No odor |
| Appearance | Off-white powder |
| Complexity | 112 |
| Dipole Moment | 0 |
| Solubility | Insoluble in water, soluble in alcohol |
Chemical Properties
- The density of dimethylglyoxime is 1.37 g/cm3.
- It has a molecular weight of 116.12 g/mol.
- The boiling point is not determined.
- Melting point for this chemical compound is 240℃ to 241℃.
- Nickel cation(Ni+2) reacts with the compound to form an insoluble red precipitate of nickel dimethylglyoxime.
Ni+2 + 2C4H8N2O2 → Ni(C4H7N2O2)2 + 2H+ - The compound reacts with ferrous sulfate and ammonium hydroxide to form a complex compound of iron and ammonium sulfate and water.
FeSO4 + 2NH4OH + 2C4H8N2O2 → Fe(C4H7N2O2)2 + (NH4)2SO4 + 2H2O
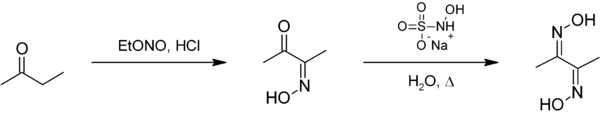
Preparation of Dimethylglyoxime
It can be prepared from butanone. First, by a reaction with ethyl nitrite to give diacetyl monoxime. The second oxime is then installed using sodium hydroxylamine monosulfonate.
Applications of Dimethylglyoxime
- The compound is used extensively in analytical chemistry for different metal ions such as platinum, palladium, etc., in the form of detecting reagent, precipitating reagent, and photometric reagent.
- It is used as a test for nickel release and used for jewelry.
- It can also be used as a specific precipitant for nickel and palladium.
Hazards
The chemical compound is hazardous as it is toxic and is a source of skin or eye irritants. It also has harmful effects if swallowed. The compound has also been observed to be flammable.
FAQs
It is a bidentate ligand chelating large amounts of metals.
The chemical compound is hazardous as it is toxic and is a source of skin or eye irritants. It also has harmful effects if swallowed. The compound has also been observed to be flammable.
Its IUPAC name is N-(3-nitrosobut-2-en-2-yl)hydroxylamine
Its chemical formula is C4H8N2O2
It is a neutral complex which is the reason for its insolubility i.e it is insoluble since there are no charges on the compound to bind and solve the ion by polar water molecules.
More Compounds
