Sodium Zincate is an inorganic compound. It is also known as Disodium Tetrahydroxy Zincate having the formula as Na2Zn(OH)4.
The inorganic compound was synthesized as byproduct in Zinc granule Reaction. It is a toxic compound.
Index
Structure & Formula
The chemical formula of Sodium Zincate is Na2Zn(OH)4 and the structure is represented as below.
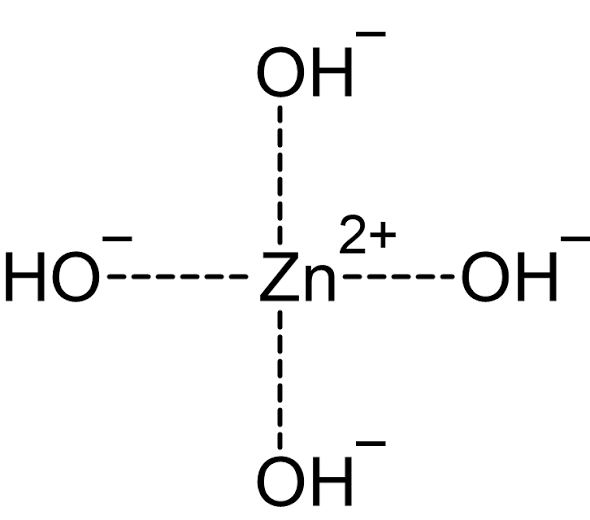
Preparation Methods
The inorganic compound is heavily toxic and there’s no purpose in real life hence no commercial method for preparation.
Lab Preparation Method
The compound is prepared in lab by dissolving Zinc or Zinc oxide or Zinc hydroxide in an aqueous solution of Sodium hydroxide (NaOH).
ZnO +H2O + 2NaOH -> Na2Zn(OH)4
Zn + 2H2O + 2NaOH -> Na2Zn(OH)4 + H2
Properties of Sodium Zincate
The physical and chemical properties of this chemical compound is discussed below.
Physical Properties
| Odour | Toxic |
| Colour | White Powder |
| Melting Point | NO DATA |
| Boiling Point | NO DATA |
| Solubility | Fairly Soluble in Water |
| Molecular Mass | 179.418 |
| Nature | Basic |
Chemical Properties
It is highly toxic and no chemical reaction is recorded up to date.
Applications
- Used to treat low levels of Zinc in living beings.
- Used to increase Solubility of cellulose at low Temperature.
Toxic Effects
- Nausea, Stomach upset, Heart burn may occur.
- Harmful to aquatic organisms.
FAQs
The chemical compound contains both ionic bonds and Covalent bonds. Ionic bond is present between Sodium and Zinc whereas Covalent bond is present between Oxygen atoms.
The compound has the formula as Na2Zn(OH)4.
Zincate is an zinc containing compound used in treatment of low level Zinc whereas Zantac contains no Zinc in it. It is used for acidity and is the most used drug in the world.
Related Topics:
