The chemical compound carbendazim belongs to a class of benzimidazoles known as 2-aminobenzimidazole wherein the primary amino group is substituted by a methoxycarbonyl group.
Index
Structure & Formula
Carbendazim has the chemical formula, C9H9N3O2. The 2D structure of the compound is shown below.
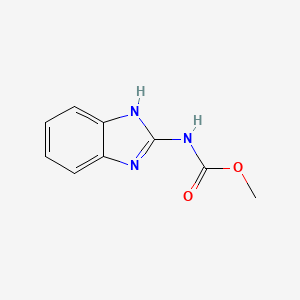
The compound has the IUPAC name, methyl N-(H-benzididazol-2-yl) carbamate.
Chemical & Physical Properties
The following are the chemical and physical properties of the chemical compound.
| Molecular Weight | 191.19 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Mass of the compound | 191.069476538 |
| Topological Polar Surface Area | 67 Å2 |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 222 |
| Isotope Atom Count | 0 |
| Is it Canonicaliized? | Yes |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| Physical appearance | Light gray or beige powder |
| Odor | Odorless |
| Melting Point | 302-307℃ |
| Solubility | < 1mg/mL at 70℉ |
| Density | 1.45 at 68℉ |
| Vapor Pressure | < 0.000000075 mm Hg at 68℉ |
Applications
- Carbendazim is used as an additive non-reactant.
- It is used in fungicide.
- The compound is used as a preservative in the paint, papermaking, and leather industries.
- It is also used as a preservative of fruits.
- Carbendazim is used as an antimicrobial in concrete, inks, sealants, textiles, etc.
Hazards
Carbendazim is hazardous to living organisms as it may cause
- Genetic defects
- Damage fertility
- Highly toxic to aquatic life
FAQs
The chemical compound must be kept in a tightly-closed container in a dry and well-ventilated environment.
The chemical compound carbendazim is manufactured by the reaction of o-phenylenediamine(C6H8N2) with methoxycarbonylcyanamide or with methoxycarbonyl isocyanide dichloride in the presence of a base.
More Organic Compounds
