The term Isotopes is important in chemistry as it refers to chemical species with the same atomic number but different masses. It has many uses in our daily life.
Index
Definition
They can be defined as follows:
“Isotopes are two or more kinds of atoms that have the same atomic number (Z) but have a different atomic mass number (A).”
In other words, they can be defined as:
“Atoms with the same number of protons (Z) but different number of neutrons (A-Z), and hence different number of nucleons (A)”
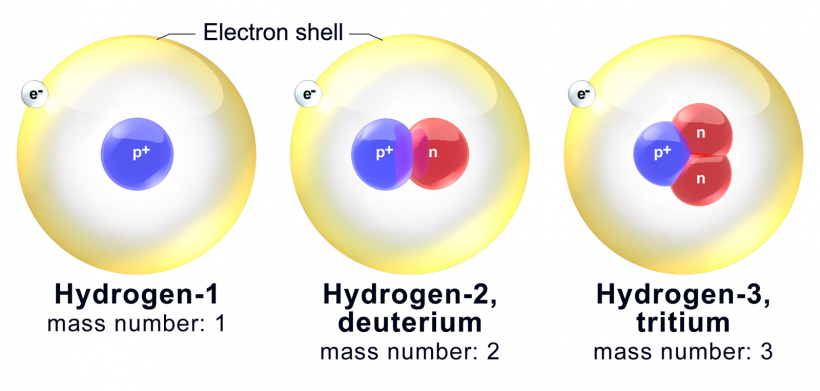
Note the same number of protons (blue) but a different number of neutrons (red). (Source)
What are Isotopes?
As we have stated above, isotopes are atoms with the same atomic number (Z), but a different mass number (A). This is because they have the same number of protons but a different number of neutrons.
As a result, isotopes of one element has the same position in the periodic table. They have different physical properties but mostly similar chemical properties.
This is because physical properties are affected by atomic mass. The heavier isotope has different properties when compared to the lighter isotope.
On the other hand, chemical properties are largely influenced by the number of electrons. This simply equals to the number of protons, which is the same for isotopes. Thus they have similar chemical properties.
Identifying Isotopes
Now, after knowing about isotope and their definitions, a major question arises is to how to identify isotopes?
They can be identified based on their physical properties. This is because they have different masses, which results in different physical properties.
One such method is the usage of mass spectrometry, which uses the difference in atomic masses to separate them.
Other methods of separation or identification of isotope are thermal diffusion (used for Uranium), and centrifugation (again depends on the masses of isotope).
In most cases, the difference in masses results in their identification.
Radioactive Isotopes
For most nuclei, there is a stable ratio of protons and neutrons. In some isotopes, this ratio is not maintained. As a result, the nucleus is unstable and can decay radioactively. Such isotopes are radioactive and are also called as radioisotopes.
They can decay via various decay modes, like alpha decay, beta decay and so on. In the process, radioactivity is observed. Many radioisotopes can be harmful to health since radioactivity is in general hazardous.
radioisotopes were studied first by Henri Becquerel, as well as the Curie couple, Marie and Pierre.
Know more on Radioactivity: Law of Radioactive Decay
Finding Average Atomic Mass
When an element has multiple isotopes, its average mass is calculated using the masses and abundances of its isotopes.
Let an element X have isotope X1, X2, … Xn and their masses be M1, M2, … Mn. Let the abundance (or per cent occurrence) of each isotope be a1, a2, … an.
Then, the formula for average mass of isotope is:
\(\text{M}_{\text{Average}} = \frac{a_1 M_1 + a_2 M_2 + a_3 M_3 + \cdots + a_n M_n}{100}\)Example
Question. Find the average atomic mass of Chlorine. It has two major isotopes, Chlorine-35 (75% abundance) and Chlorine-37 (25% abundance).
Solution. Here, M1 = 35, M2 = 37; a1 = 75%, a2 = 25%. As a result, we have, from the above formula,
\(\begin{align}
\text{M}_{\text{Average}} & = \frac{a_1 M_1 + a_2 M_2 + a_3 M_3 + \cdots + a_n M_n}{100} \\
& = \frac{75 \times 35 + 25 \times 37}{100} \\
& = \frac{3550}{100} \\
\text{M}_{\text{Average}} & = 35.5
\end{align}
\)
Thus the average atomic mass of Chlorine is 35.5 atomic mass units.
FAQs
These are atoms or nuclei with the same atomic number (Z) but different atomic mass (A). They have the same number of protons but different number of nucleons.
Some useful applications are:
– Carbon-14, a radioactive isotope of carbon, is used in estimating the age of some fossils.
– Uranium-235 is an isotope of Uranium that is used in nuclear fission reactions for power plants.
– Iodine-131 is used to treat cancer of the thyroid gland.
The stable isotopes of carbon are Carbon-12 (~99% abundance) and Carbon-13 (~1% abundance). The only other natural isotope is Carbon-14, but it decays radioactively.
Isotopes are produced in two ways:
Naturally, through the decay of heavier nuclei
Artificially, by the bombardment of nuclei with a range of charged particles.
Related Topics:
1. Isobars
2. Isotones
3. Isodiaphers
