Isodiaphers are nuclei with different neutron (n) and proton (p) numbers, but the same neutron excess. The term isodiapher is used in nuclear physics and chemistry, along with related terms like isotopes and isobars.
Index
What are Isodiaphers
Nuclides, or nuclei, consist of protons and neutrons. The number of neutrons is often denoted by \(\mathbf{N}\) while the number of protons is simply the atomic number, \(\mathbf{Z}\).
Isodiaphers in chemistry are defined as two or more nuclei, which have the same neutron excess. In other words, the difference \(\mathbf{N}-\mathbf{Z}\) is the same for isodiapher.
Applications
The term Isodiapher is a useful way to refer to nuclei who maintain the same \((\mathbf{N}-\mathbf{Z})\) value. This occurs in the case of alpha decay, where two neutrons and two protons leave the parent nucleus, leaving the same \((\mathbf{N}-\mathbf{Z})\) value for the daughter nucleus.
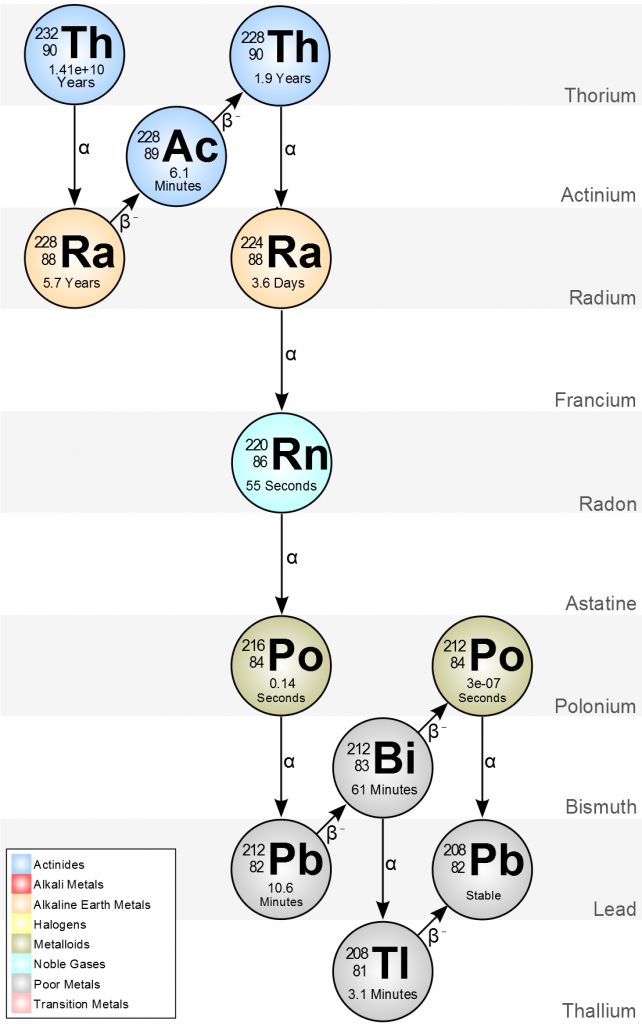
Example
Example 1. Which set of three nucleii are isodiaphers: \(^{13}_{6}\mathrm{C}\), \(^{14}_{7}\mathrm{N}\) and \(^{15}_{8}\mathrm{O}\) or \(^{13}_{6}\mathrm{C}\), \(^{15}_{7}\mathrm{N}\), and \(^{17}_{8}\mathrm{O}\)?
Let us consider the first set. We have,
| Nucleus | Mass Number \(\boldsymbol{(A)}\) | Protons \(\boldsymbol{(Z)}\) | Neutrons \(\boldsymbol{(N = A – Z)}\) | Neutron Excess \(\boldsymbol{(N – Z)}\) |
| \(^{13}_{6}\mathrm{C}\) | 13 | 6 | 7 | 1 |
| \(^{14}_{7}\mathrm{N}\) | 14 | 7 | 7 | 0 |
| \(^{15}_{8}\mathrm{O}\) | 15 | 8 | 7 | -1 |
Clearly, the neutron excess is NOT the same. So the first set are not isodiapher. However, they are isotones, as number of neutrons is same.
Let us now see the second set.
| Nucleus | Mass Number \(\boldsymbol{(A)}\) | Protons \(\boldsymbol{(Z)}\) | Neutrons \(\boldsymbol{(N = A – Z)}\) | Neutron Excess \(\boldsymbol{(N – Z)}\) |
| \(^{13}_{6}\mathrm{C}\) | 13 | 6 | 7 | 1 |
| \(^{15}_{7}\mathrm{N}\) | 15 | 7 | 8 | 1 |
| \(^{17}_{8}\mathrm{O}\) | 17 | 8 | 9 | 1 |
In this set, the neutron excess is always the same, and is 1. Thus the second set are isodiapher.
Example 2. Are the following isodiaphers? \(^{228}_{90}\mathrm{Th}\), \(^{224}_{88}\mathrm{Ra}\), \(^{220}_{86}\mathrm{Rn}\) and \(^{216}_{84}\mathrm{Po}\).
We again tabulate the relevant numbers and examine.
| Nucleus | Mass Number \(\boldsymbol{(A)}\) | Protons \(\boldsymbol{(Z)}\) | Neutrons \(\boldsymbol{(N = A – Z)}\) | Neutron Excess \(\boldsymbol{(N – Z)}\) |
| \(^{228}_{90}\mathrm{Th}\) | 228 | 90 | 138 | 48 |
| \(^{224}_{88}\mathrm{Ra}\) | 224 | 88 | 136 | 48 |
| \(^{220}_{86}\mathrm{Rn}\) | 220 | 86 | 134 | 48 |
| \(^{216}_{84}\mathrm{Po}\) | 216 | 84 | 132 | 48 |
As we see, the neutron excess is the same for all 4 nuclei, and they are thus examples of isodiaphers. In fact, they are obtained in alpha decay of \(^{228}_{90}\mathrm{Th}\).
Related:
1. Isobars
2. Isotopes
3. Isotones
FAQs
Nuclei with the same neutron excess \(\mathbf{N}-\mathbf{Z}\) are called isodiaphers.
No, isotones and isodiaphers are not the same.
Isotones are nuclei with the same number of neutrons \(\mathbf{N}\).
Isodiaphers are nuclei with the same neutron excess, i.e. \(\mathbf{N}-\mathbf{Z}\).
A nuclide is an atomic species with a given number of protons and neutrons, and a given state of nucleus. It is a term used in nuclear physics.
Isodiaphers come up naturally in alpha decay chains. Thus the term can be used to discuss them.
