Born Haber Cycle also called as Born-Fajans-Haber Cycle or Born-Haber Process is a visual representation of various reactions and their corresponding energies, which leads to an overall reaction and overall reaction energy.
The Born-Haber process is used to find the energy of formations of ionic compounds. Most of the time, the formation of ionic compounds from their elemental components requires various intermediate processes. The Born-Haber process attempts to simplify the evaluation of the overall reaction by providing a visual aid that is easier to understand.
Index
History
The Born-Haber process is named after the two famous German Scientists Max Born and Fritz Haber. The cycle was developed by contribution from both of them in the year 1919. A Polish-American Chemist, Kazimierz Fajans, developed the same cycle independently.
What is Born Haber Cycle
The Born Haber cycle is mainly used to calculate the lattice enthalpy of various ionic compounds, otherwise, it is difficult to find.
Lattice Energy is the energy released when free anions and cations come closer to form a lattice structure. This energy is due to the electrostatic force of attraction.
The cycle involves the use of Hess’ law extensively to find this lattice energy.
Hess’ Law:
Hess Law of constant heat summation states that for a multi-stage reaction with various intermediate reactions, the enthalpy change for the overall reaction is the sum of the enthalpy changes of the intermediate reactions.
Now let’s try to understand how to use a Born-Haber process by an example.
Born-Haber Cycle: Explained by Example
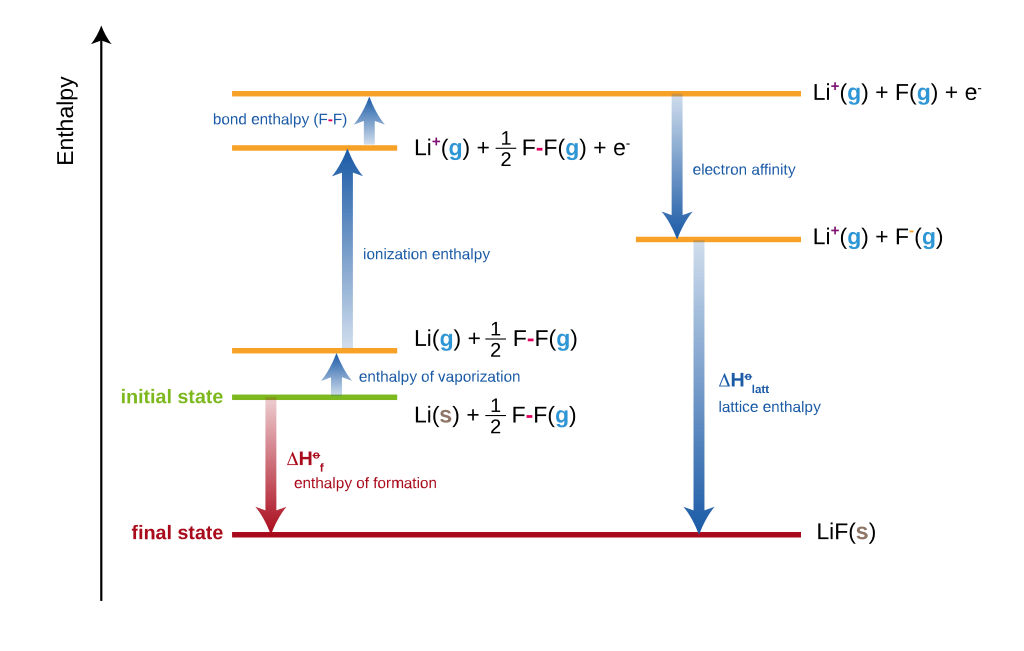
Let’s understand how to use Born Haber cycle by drawing the cycle of Lithium Fluoride and write down the Hess’ Law for it.
Step 1: Write the overall reaction of the formation of Lithium Fluoride from the individual elements.
\(2Li(s) \ + \ F_2(g) \rightarrow 2LiF(s)\)Now, balance the reaction using fractions such that we get one mole of product.
Here, multiply the equation by half:
\(Li(s) \ + \ \frac{1}{2}F_2(g) \rightarrow LiF(s)\)
Step 2: List out all the intermediate steps of the overall reaction.
- Here, Li is in solid states as a reactant. So first, it needs to be converted to a gaseous state (Enthalpy of Vaporization).
- F2 also needs to be broken down into individual F (F-F Bond Enthalpy).
- To produce Li+ from Li, electrons are expelled out (Ionization Enthalpy).
- To produce F– ions from F atoms, electrons are required (Electron Affinity).
- Lattice Energy is released when Li+ and F– in their gaseous states, arrange themselves in a lattice structure to produce solid LiF.
- When LiF(s) is subjected to disintegration and Li(s) and \(\frac{1}{2}\)F2(g) is produced, a lot of energy is required (Enthalpy of Formation of LiF(s)).
Step 3: Obtain Born Haber Cycle from above information
Draw a vertical arrow denoting the Enthalpy or Energy axis.
Draw a long horizontal line denoting the energy level of the product LiF(s).
Construct and connect various levels which require energy, in the left side of the LiF(s) level, with increasing energy.
Next, connect and construct the various other levels which give out energy.
In this way, obtain a closed-loop cycle. This cycle is called the Born-Haber cycle.
Step 4: Use Hess’ Law as follows:
For a whole cycle, the energy change is zero.
\(0= \Delta H_{f} + \Delta H_{vap} + \frac{1}{2} \Delta H_{F_2} + I E_{\mathrm{Li}} – EA_{\mathrm{F}} + \Delta H_{L}\)
Where,
\(\Delta H_{f} \rightarrow\) Enthalpy of Formation of LiF
\(\Delta H_{vap} \rightarrow\) Enthalpy of Vaporization of Li
\(\Delta H_{F_2} \rightarrow\) Bond Enthalpy of F-F
\(IE_{\mathrm{Li}} \rightarrow\) Ionisation Enthalpy of Li
\(EA_{\mathrm{F}} \rightarrow\) Electron Affinity of F
\(\Delta H_{L} \rightarrow\) Lattice Enthalpy
Using this equation, find the desired unknown quantity.
Some More Examples
More examples on Born Haber process to grasp the concept better:
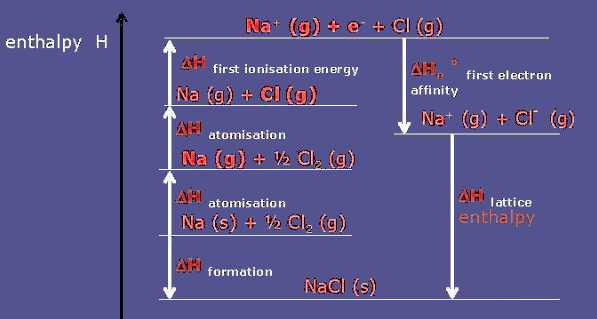
Born-Haber Process of Sodium Chloride (NaCl):
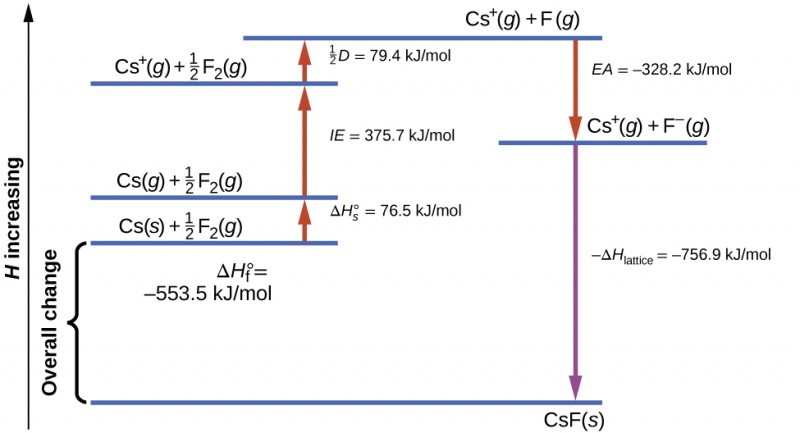
Born-Haber Process of Caesium Fluoride (CsF):
FAQs
Born-Haber cycle also called as Born-Fajans-Haber cycle or Born-Haber process is a visual representation of various reactions and their corresponding energies, which leads to an overall reaction and overall reaction energy.
The process can’t be used for compounds which are strictly not ionic in nature. All Alkali Halides are such compounds. Compounds of Alkali and some alkaline earth metals are also ionic, with oxygen as the anionic atom.