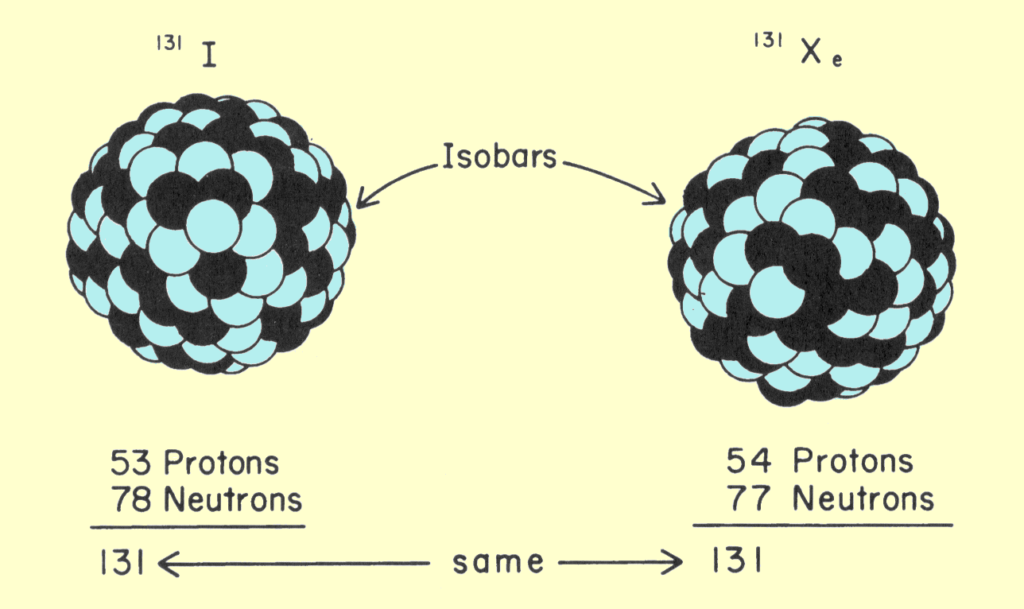
Isobars are atoms or nuclei which have the same number of nucleons. In other words, they have the same atomic mass. The term was coined in 1918 by Alfred Walter Stewart.
Index
Isobars Definition
The isobar definition can be stated as follows:
“These are atoms or nuclei which have the same atomic mass number, A. They have the same number of nucleons, i.e. protons plus neutrons.”
Related Topics:
Examples of Isobars
Some examples of isobar are as follows:
- Argon-40 (\(^{40}_{18}\mathbf{Ar}\)), Potassium-40 (\(^{40}_{19}\mathbf{K}\)), and Calcium-40 (\(^{40}_{20}\mathbf{Ca}\))
All these elements are isobar. They have the same atomic mass number, 40. The number of protons and neutrons are different, however. - Carbon-14 (\(^{14}_{6}\mathbf{C}\)), Nitrogen-14 (\(^{14}_{7}\mathbf{N}\)), and Oxygen-14 (\(^{14}_{8}\mathbf{O}\)) also are isobar, with common atomic mass number A = 14.
Applications
Isobars often are studied in nuclear physics and radioactivity. They naturally occur in the study of beta decay. The term isobar is a useful classifying criterion.
FAQs
These are chemical species (atoms or nuclei) that have the same atomic mass number (A). They have the same number of nucleons, that is protons and neutrons.
These are two commonly used terms in chemistry.
Isotopes have the same atomic number (Z) but a different mass number (A).
E.g.: Chlorine-35 and Chlorine-37.
Isobars have the same mass number (A) but a different atomic number (Z).
E.g.: Argon-40 and Calcium-40.
Isobar in thermodynamics is different from isobar in nuclear physics.
In nuclear physics, they are nuclei with the same mass number.
In thermodynamics, they are graphs representing states of matter at constant pressure.
They occur in beta decay when a proton is converted to a neutron or vice versa. As we can see, the number of nucleons remains the same. The daughter nucleus is an isobar of the parent nucleus.
