Moseley’s Law tells us that the atomic number (and not atomic weight) is the fundamental property of elements. The law basically connects the frequency of an emitted X-ray and the atomic number of an element.
Index
History
The law was discovered and published by the English physicist Henry Moseley in 1913-1914.

The historic periodic table was roughly ordered by increasing atomic weight, but in a few famous cases, the physical properties of two elements suggested that the heavier element preceded the lighter element.
Henry Moseley and other physicists used x-ray diffraction to study the elements, and the results of their experiments led to organizing the periodic table by Atomic Number or Proton count.
Using this law, Moseley arranged Potassium(K) and Argon(Ar), and Nickel(Ni) in a proper way in Mendeleev’s periodic table.
What is Moseley’s Law?
Statement of Moseley’s Law: “The square root of the frequency of the x-ray emitted by an atom is proportional to its atomic number.”
The frequency of a spectral line in the characteristics X-ray spectrum varies directly as the square of the atomic number of the element emitting it.
The accurate mathematical equation between frequency and atomic number or the equation of Moseley’s laws:
\(\nu = a(Z – b)\)where, \(a = 4.97107\), \(b = 1\), \(\nu = \mbox{Frequency for }k_{\alpha}\mbox{ lines}\), \(Z = \mbox{atomic number}\)
Contributions of Moseley’s Law
As Henry Moseley was studying the \(k_{\alpha}\) graphs(when an electron vacancy in the K-Shell is filled by an electron from the L-Shell, the wavelength of the emitted photon is called k Lines) of different metals, he found a strange relationship between the lines and the atomic number.
Thus when he plotted a graph between \(\sqrt{\nu}\) (root over nu(\(\nu\))-which is the symbol of frequency for the \(k_{\alpha}\) lines ) and Z(Atomic number), he saw a straight line as follows:-
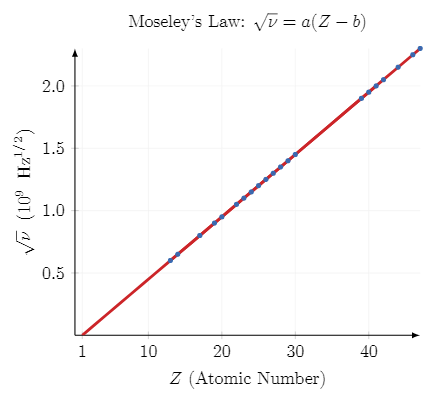
He proposed a formula \( a(Z – b)\) which was completely based on his observations.
We can understand that the Periodicity of the periodic table should probably be based on Atomic Number and not atomic Mass.
Before this, chemists believed that elements should be arranged on the basis of their atomic mass. For Example, Cobalt(Atomic mass = 58.93) was placed before Nickel(Atomic Mass = 58.69) . Clearly, Ni has a lower Atomic mass and so the arrangement is wrong.
But if we consider the Atomic Number of Co(Atomic Number = 27) and Ni(Atomic Number = 28), The arrangement is absolutely fine.
Thus, Moseley’s Law contributed to the modern-day arrangement of the periodic table on the basis of Atomic Number and not Atomic Mass.
Moseley’s Law Derivation
So according to Henry Moseley, We have \(\nu = a (Z – b) …(1)\)
Let a transitive occur from \(n_1\) to \(n_2\) state as per Bohr’s theory. The energy of an emitted photon is
\(h\nu = RChz^{2}(\frac{1}{n^2_1} – \frac{1}{n^2_2})\)
where,
\(n_1\) = quantum number of final energy level
\(n_2\) = quantum number of initial energy level
\(\nu\) = frequency for the \(k_{\alpha}\) lines
\(z\) = Atomic number
\(h = 6.63 * 10^{-34} Js\)
\(R\) = Rydberg constant
\(C\) = Constant
\(\nu = RCz^{2}(\frac{1}{n^2_1} – \frac{1}{n^2_2})\)
\(RC(\frac{1}{n^2_1} – \frac{1}{n^2_2}) = a^2\)
\(\nu = a^2 z^2\)
\(\sqrt{\nu} = az … (2)\)
Correction:- For \(k_{\alpha}\) emission, one of the two k electrons is first emitted leaving a single electron in the K shell. The negative charge of thin residual electrons partially screens the nuclear charge(Z*e) of the atom.
Now, due to the effective Coulomb force, it becomes (Z-1)e
Now, \(Z^2 \rightarrow (Z-1)^2\)
So, \(\nu = a^2(Z-1)^2\)
For \(k_{\alpha}\) emission, \(n_1\) = 1; \(n_2\) = 2
\(\nu = RC(\frac{1}{1}- \frac{1}{4})(Z-1)^2\)
\(\nu = \frac{3}{4}RC(Z-1)^2\)
\(\sqrt{\nu} = \sqrt{\frac{3}{4}RC}.(Z-1) … (3)\)
From comparing (1) and (3), we get \(a = \sqrt{\frac{3}{4}RC}\) and \(b = 1\).
Applications of Moseley’s Law
- The law led to the discovery of new elements like Hafnium (72), Technetium (43), Rhenium (75), etc.
- Using the concept of Atomic Number instead of Atomic Mass for arranging elements in the periodic table helped a lot in solving many discrepancies.
Examples
Question 1. If \(\lambda_{Cu}\) is the wavelength of \(k_{\alpha}\) X-ray line of copper (atomic number 29) and \(\lambda_{Mo}\) is the wavelength of the \(k_{\alpha}\) X-ray line of molybdenum (atomic number 42), the ratio \(\lambda_{Cu} / \lambda_{Mo}\) is close to
- 1.99
- 2.14
- 0.50
- 0.48
Solution. The wavelength of \(k_{\alpha}\) X-ray line is related to atomic number Z by Moseley’s Formula
\((\frac{1}{\lambda}) = RC(Z-1)^2 (\frac{1}{1^2} – \frac{1}{2^2})\)
\((\frac{1}{\lambda}) = \frac{3}{4}RC(Z-1)^2\)
Substitute the value of Z to get
\(\frac{\lambda_{Cu}}{\lambda_{Mo}} = \frac{(Z_{Mo} -1)^2}{(Z_{Cu} – 1)^2} = \frac{{41}^2}{{28}^2} = 2.14\)
The elements with higher atomic number (molybdenum in this example) give high energy X-rays (short wavelengths).
Question 2. Which of the following statements is wrong in the context of X-rays generated from an X-ray tube?
- The wavelength of characteristic X-rays decreases when the atomic number of the target increases.
- The cut-off wavelength of the continuous X-rays depends on the atomic number of the target.
- Intensity of the characteristic X-rays depends on the electrical power given to the X-ray tube.
- The cut-off wavelength of the continuous X-rays depends on the energy of the electrons in the X-ray tube.
Solution. The frequency of characteristic X-rays is related to atomic number Z by Moseley’s law,
\(\sqrt{\nu} = a (Z – b)\)
which gives,
\(\lambda = \frac{c}{\nu} = \frac{c}{a^2(Z-b)^2}\)
Thus, the wavelength of emitted X-rays decreases with an increase in Z.
The cut-off wavelength of continuous X-rays corresponds to the maximum energy of electrons in an X-ray tube. It is given by
\(hc/\lambda = eV\)
Where V is the accelerating potential. The intensity of X-rays depends on the number of electrons striking the target per second, which, in turn, depends on the electrical power given to the X-ray tube as the energy of each electron is eV.
FAQs
Moseley’s Law is important as it proved that the atomic number is a more fundamental property of elements and not Atomic mass.
Moseley’s Law is known for the telling atomic number as a fundamental property by showing the proportionality between the frequency of X-ray emitted by an element and its atomic number.
When there is a vacancy in the K shell and it is filled by an electron from the L shell, the energy/wavelength of the emitted photon is called the K-alpha spectral line and when it is filled by an electron from M shell it is called K-beta spectral line.
