The Schottky defect is a type of point defect that can occur in crystalline solids.
It is named after the German physicist Walter H. Schottky, who first described this phenomenon in 1932.
Schottky was studying the properties of ionic crystals, such as sodium chloride. He observed that they exhibited a decrease in density when heated. He proposed that this decrease in density was due to the creation of vacancies in the crystal lattice, where an equal number of cations and anions were missing.
Schottky’s proposal was later supported by X-ray diffraction studies, which showed a decrease in the intensity of diffraction peaks in heated crystals. This decrease in intensity was interpreted as evidence of missing ions, which confirmed the existence of Schottky defects.
Index
Explanation
The Schottky defect is a special case of a vacancy defect. It involves an equal number of cations and anions missing from the lattice, and therefore the crystal remains electrically neutral.
The formation of Schottky defects is favored in ionic crystals that have a high coordination number, meaning that each ion has a large number of neighboring ions.
However, in the case of a Schottky defect, a pair of oppositely charged ions, typically one cation and one anion, are missing from the lattice. This creates a vacancy defect where two ions are absent in the crystal, leaving behind two adjacent lattice sites that are empty.
Schottky defects can occur in ionic crystals when the crystal is heated or exposed to radiation, causing some ions to be dislodged from their lattice sites. They can also occur as a result of intrinsic defects in the crystal. Such as impurities or imperfections in the crystal structure.
Schottky defects can have important effects on the physical properties of ionic crystals.
For example; They can alter the crystal’s electrical conductivity, as the missing ions create pathways for electrical current to flow through the crystal. They can also affect the crystal’s optical properties, such as its ability to absorb or transmit light.
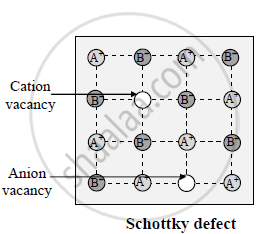
Schottky defects can occur in many different ionic crystals.
Example
An example of a material where Schottky defects are commonly observed is Sodium Chloride (NaCl), also known as table salt.
In a perfect NaCl crystal, every sodium ion (Na+) is surrounded by six chloride ions (Cl-), and every chloride ion is surrounded by six sodium ions.
However, due to various reasons such as high temperatures or pressures, some ions in the crystal lattice can be removed from their original positions, creating a vacancy defect.
In the case of the Schottky defect in NaCl, an equal number of sodium and chloride ions are missing from the lattice. It creates a pair of vacant sites. The formation of these vacancies does not change the overall charge of the crystal since the same number of positive and negative ions are missing.
The presence of Schottky defects in NaCl leads to a reduction in density and an increase in electrical conductivity. This is because the vacant sites can act as electron donors, allowing the movement of charged species through the crystal.
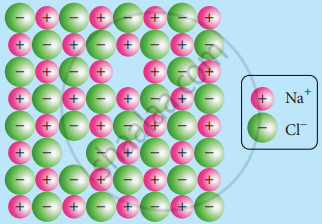
Schottky defects are also commonly observed in other ionic crystals such as potassium chloride (KCl), and magnesium oxide (MgO).
Applications
- Diode: Schottky defects can be used as a channel between two contacts in a diode. It is used in the creating a Schottky contact as well.
- Solar cells: Schottky defects are also used in solar cells.
- Transistor: In the semiconductor industry, the Schottky defect is used to control the conduction between a p-type and n-type semiconductor.
Related Articles:
1. Schottky Diode
2. Frenkel Defect
FAQ’s
The Schottky defect is a vacancy defect that lowers the density of the solid crystal.
Alkali metals show Schottky defects because of the larger size of the cations of the alkali metals, due to the large size the cations cannot fit into the interstitial sites.
The Schottky defect is not observed in ZnS.
No, this is false. While Schottky defects are often described as occurring in pairs, they can occur singly also in some cases.
No, this is also false. Schottky defects do affect the stoichiometry of a compound, as they involve the removal of one cation and one anion from the crystal lattice. This leads to a decrease in the overall number of ions in the compound.
