The Octet Rule is one of the most basic and fundamental rules in the chemical bonding of atoms. It is a theory that describes exactly why elements(except d- and f-block elements) participate in chemical bonding.
All elements(except d- and f-block elements) want to become stable and unreactive by achieving the electronic configuration of a noble gas that has 8 electrons in its outermost shell, this is the octet rule.
Index
History
The octet rule’s foundations were laid when Richard Abegg first observed that the difference between the maximum and minimum coordination numbers of an element often resulted as eight.
Gilbert N. Lewis took this observation of Richard to develop his own theory of a rule of eight and a model called the cubical atom model.
Irving Langmuir was the scientist who refined these concepts(cubical atom model and rule of eight) of Lewis as the cubical octet atom and octet theory. The octet theory that Langumir developed later evolved into the now known octet rule.
Another important contribution toward the development of the octet rule is the theory proposed by Walter Kossel and Lewis, called the electronic theory of valency.
This theory essentially says that chemical bonds are formed between various atoms, in order to achieve a noble gas configuration. It is based on the fact that noble gases participate less in chemical reactions.
Octet Rule Explained
The octet rule states that the elements with eight electrons in the valence shell tend to be more stable and so, elements form bonds with other elements in such a way that they achieve this state.
This rule is applied only to those elements whose valence shell electrons are only in s- and p-orbitals.
For Example, if we consider Ar whose atomic number is 18 has 8 electrons in the valence shell, so the elements near Ar try to attain its configuration through chemical bonding.
This rule is usually identified in chemical bonding by representing the bonding atoms using the Lewis electron-dot structure.
Limitations of Octet Rule
There are a few basic limitations of octet rule, these are:
- Reactive intermediates like nitrene, carbene and free radicals do not follow this rule as they are. So, these intermediates form bonds in order to gain an octet.
- There are odd electron compounds which though stable do not follow the rule.
There are two kinds of odd electron compounds:- Compounds having greater than 8 electrons surrounding them. For example, PF₅.
- Compounds having less than 8 electrons surrounding them. For example, B₂F₆.
- The elements whose valence shell has a maximum of only 2 electrons, like that of Hydrogen(H) or Helium(He) are stable without following the octet rule. For them, the duplet rule is valid.
- Transition and inner transition elements do not follow this rule. There are plenty of stable transition elements with more than 8 electrons in their valence shell.
Odd Electron Species
The odd electron species of elements sometimes form unique bonds called three electron bonds between them in order to achieve an octet in their valence shell. The three electron bonds are formed by sharing of three electrons among two atoms.
For Example, an oxygen molecule shows paramagnetic nature which cannot be explained through VBT.
So, Pauling proposed the existence of two-three electron bonds and one covalent bond in an oxygen molecule.
Here, the three electron bonds have one unpaired electron each, validating the paramagnetic nature of the molecule. The total bond order is two which is in accordance with experimental results.
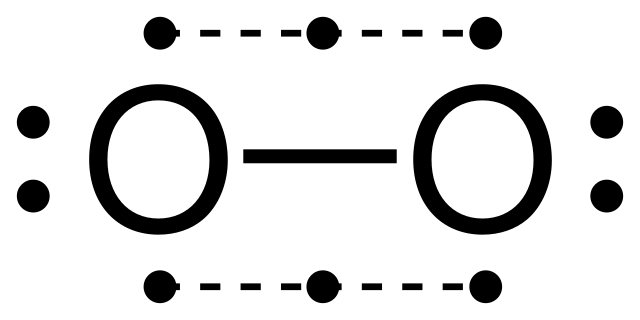
There are also cases of hyper valency in the p-block elements during bond formation as mentioned before. For example, in PF5, the central atom P is surrounded by 10 electrons, which is a violation of this rule.
This phenomenon is explained by hybridization theory where one s, three p and one d orbitals merge to form an sp³d hybrid orbital with 2 electrons in each arm making 10 in total.
There is an alternate explanation to this hyper valency of P which shows that it follows the octet rule. According to the Molecular Orbital Theory (MO Theory), there are only four bonding MOs and the other one is a high energy antibonding MO. So, effectively there are only 8 electrons involved in bonding, hence it follows the octet rule.
Applications
- This rule explains the formation of covalent and ionic bonds.
- Explains the high reactivity of elements close to noble gases.
- Explains the high stability and inertness of the noble gases.
- Helps in predicting the properties of p-block elements.
Example
Question. Show that MgCl₂ bonds according to octet rule.
Answer. The electronic configuration of,
Mg: 1s2 2s2 2p6 3s2
Cl: 1s2 2s2 2p6 3s2 3p5
Lewis electron dot structure of MgCl2 is:
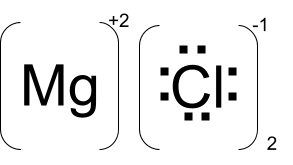
As we can see, 2 atoms of chlorine bond with one atom of magnesium.
Here, transfer of electrons takes place because the ionization enthalpy of magnesium is quite low.
Two electrons are lost from magnesium because it attains Neon configuration in doing so, which is its nearest noble gas. From this, we can say that the conversion of Mg to Mg⁺² is in accordance with the octet rule.
Now if we consider chlorine, it gains the electrons lost by magnesium. The electron gain enthalpy of chlorine is highly negative which means it becomes stable by losing energy after gaining an electron.
After gaining an electron chlorine attains the configuration of Argon which is an inert gas. The above two facts prove that the gaining of the electron by chlorine is in accordance with the octet rule.
From the above two explanations of Mg and Cl, it is clear that the formation of ionic bond in MgCl2 is due to octet rule.
FAQs
Octet rule relates the stability of the atom of an element to the number of electrons in its valence shell. It is a rule which can be used to find the relative stability and various other properties of an element.
Some examples of the limitations of the octet rule:
1. Hydrogen
2. Helium
3. Lithium (Looses one electron to attain duplet configuration of helium)
4. Phosphorus
1. There are electron deficient compounds like B2F6, A2Cl6, etc and also electron excess compounds like PF5, SF5, etc.
2. Transition elements do not follow the octet rule.
3. Reaction intermediates like free radicals do not follow the octet rule.




