Ionization Enthalpy is the energy used for the withdrawal of an electron from its gaseous atom or ion.
An atom or molecule’s first ionizing force (\(E_i\)) is the energy needed to remove one mole of electrons from one mole of separated gaseous atoms or ions.
Index
More About Ionization Enthalpy
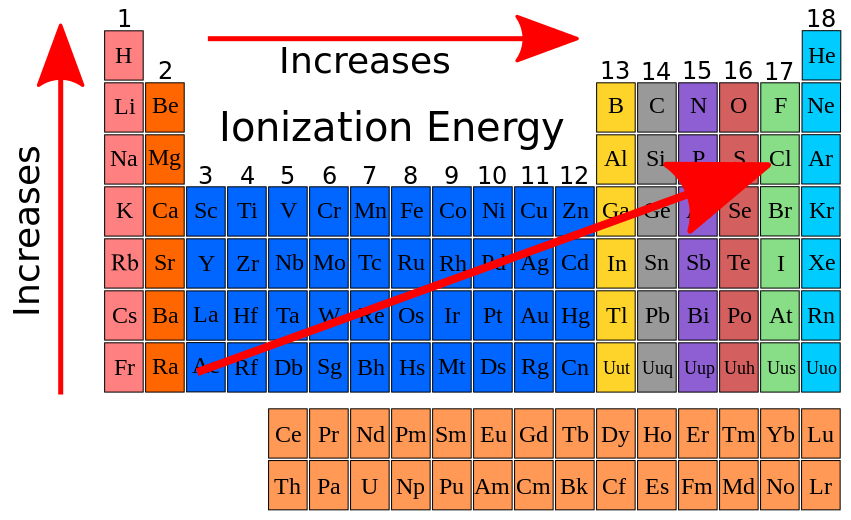
In periodic table;
- Ionization energy increases from left to right.
- It decreases from top to bottom.
Unit of ionisation Enthalpy is KJ mol-1.
The first ionization energy of element A is the energy required by an atom to lose an electron & form A+ ion.
\(A(g) \rightarrow A^{+}(g) + e^-\)
Similarly, the second ionization energy is;
\(A^{+}(g) \rightarrow A^{+2} + e^-\)
Ionisation enthalpy will always be positive, as one needs to provide a certain amount of energy to remove an electron from an atom or ion.
The second frequency of ionization will always be higher than the first ionization energy.
Learn about a related topic Electron Gain Enthalpy
Factors Affecting Ionization Enthalpy
There are certain factors that affects the ionisation enthalpy of any gaseous atom or ion. Let us look at some of the main factors.
Penetration Effect
The penetration effect is due to the penetration of nuclear charge through the orbitals which attracts the outermost shell’s electrons.
Thus, the order of penetration power is;
2s > 2p > 3s > 3p > 4s > 3d and so on… .
Shielding Effect
It is the effect in which the inner electrons develop a shield for the electrons in outer shells; this does not allow appropriate nuclear charge towards the outermost shell’s electrons.
The effective nuclear charge can be given as:
\(Z_{eff} = Z – S\)
Here,
\(Z_{eff}\) = Effective nuclear charge
\(Z\) = Actual nuclear charge
\(S\) = Screening constant
Electronic Configuration
Elements or atoms having fully or half-filled orbitals are more stable than others (octet or duplet stability).
Hence, if we try to remove an electron from these orbitals, it makes them less stable. We need more amount of energy to remove an electron from these orbitals. Thus the ionization energy is higher.
General Periodic Trends
- Ionization enthalpy increases from left to right. (Across a period)
As we move towards the right, the atom’s radius decreases, increasing the attractive force between the nucleus and the outermost electron.
- Ionization enthalpy decreases from top to bottom. (Across a group)
As we move down a group, the number of shells increases leading to the increase in the size of the atom, increasing the shielding & decreasing attractive force between the nucleus and the valence shell’s electron.
FAQs
Energy used for the withdrawal of an electron from its gaseous atom or ion is known as Ionization enthalpy.
\(Z_{eff}\) is calculated by;
\(Z_{eff} = Z – S\), where Z is the atomic number and S is the number of shielding electrons.
It is the effect in which the inner electrons develop a shield for the electrons in outer shells.
The shielding effect of different orbitals is as follows:
s orbital’s > p orbital’s > d orbital’s > f orbital’s.
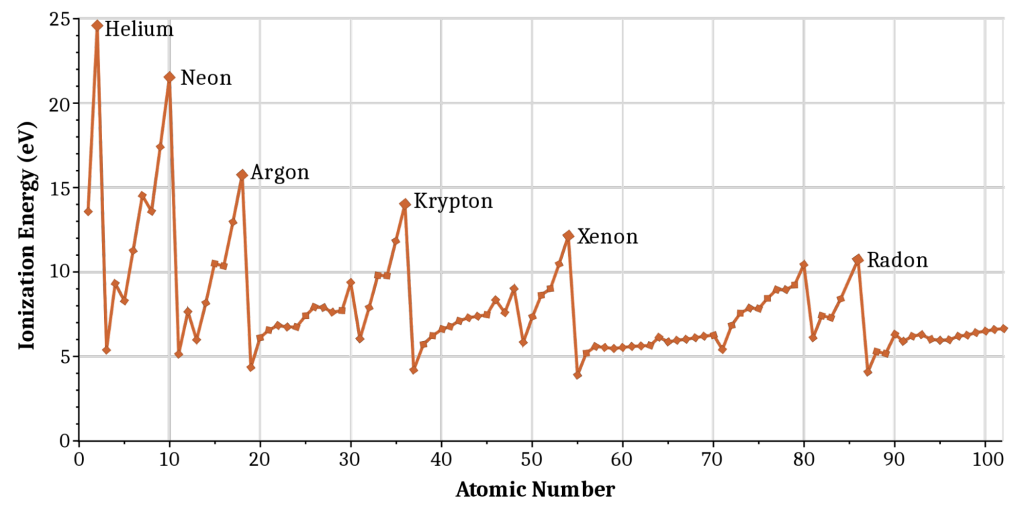
Helium(He) of group 18 has the largest first ionization enthalpy, while Francium(Fr) has one of the lowest.
