The Aufbau Principle dictates how electrons are filled in the atomic orbitals of an atom in its ground state.
Index
What is the Aufbau Principle?
The Aufbau principle states that, hypothetically, electrons orbiting one or more atoms fill the lowest available energy levels before filling higher levels (e.g., 1s before 2s). In this way, the electrons of an atom, molecule, or ion harmonize into the most stable electron configuration possible.
Aufbau is a German noun that means construction or “building-up”. It is sometimes called the building-up principle or the Aufbau rule.
The following diagram shows the order in which atomic orbitals are filled.
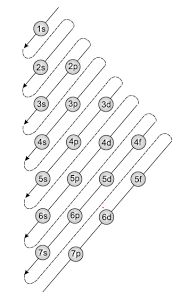
The Aufbau principle can be used to understand the location of electrons in an atom and their corresponding energy levels.
Characteristics of Aufbau Principle
According to the principle:
- Electrons first occupy orbitals with the lowest energy.
- The order in which orbital energy increases can be determined using the rule (n+l), where n is the main quantum number and l is the azimuthal numbers.
- The lower values (n+l) correspond to lower orbital energies. If two orbitals share equal values (n+l), the lower n value orbital is said to have less energy associated with it.
- The order in which the orbitals are filled with electrons is 1s, 2s, 2p, 3s, 3p, 4d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Exceptions of the Aufbau Principle
- The electronic configuration of chromium is [Ar]4s13d5 and not [Ar]4s23d4 (as the Aufbau principle suggests). This exception is attributed due to several factors such as the increased stability provided by half-filled subshells and the relatively small energy gap between the 3d and 4s subshells.
- Half-filled subshells have lower electron-electron repulsions in the orbitals, increasing stability. Similarly, subshells also increase the stability of the atom. Therefore, the electron configurations of certain atoms disobey the Aufbau rule.
- Copper is another exception to this principle with an electronic configuration corresponding to [Ar]4s13d10. This can be stability provided by a fully filled 3D subshell.
Electronic Configuration Using the Aufbau Principle
Writing the electronic configuration of Sulphur:
The atomic sulphur number is 16, implying that it holds a total of 16 electrons.
According to the Aufbau principle, two of these electrons are present in subshell 1s, eight of them are present in subshell 2s and 2p, and the rest are distributed in subshells 3s and 3p.
Therefore, the electronic configuration of sulphur can be written as 1s22s22p63s23p4.
Examples
Question 1. The maximum number of electrons in a shell is given by ___.
- 2n2
- 2n3
- 2n1
- 2n0
Answer. 1
Question 2. Ni2+(atomic number 28) cation has ___ unpaired electrons.
- 0
- 4
- 6
- 2
Answer. 2
Question 3. Which of the following orders is the correct order that electrons would fill the subshells?
- 1s, 2s, 3s, 4s, 5s
- 1s, 2s, 2p, 3s, 3p
- 3s, 3p, 2p, 2s, 1s
- 1s, 2s, 3s, 1p, 2p, 3p
Answer. 2
FAQs
Half-filled and completely filled d and f subshells add stability to atoms, so the d and f block elements don’t always follow the Aufbau principle
A specific version of the Aufbau principle known as the nuclear shell model is used to predict the configuration of protons and neutrons in an atomic nucleus.
The “n” and “l” in the (n + l) rule are the quantum numbers which are used to specify the state of a given electron orbital in an atom.
Here n is the principal quantum number and is related to the size of the orbital and l is the angular momentum quantum number and is related to the shape of the orbital.
