The charge of an electron is \( e = – 1.6021766 \ \times \ 10^{-19} \ Coulomb \). The charge of an electron is a fundamental constant of physics.
Charge of an Electron is \( e = – 1.6021766 \ \times \ 10^{-19} \ Coulomb \)
Its charge is unaffected by the motion of the particle. The electronic charge is negative. This means that an electron moves opposite the direction of the electric field.
Index
History
The charge of an electron was first determined using Millikan’s oil drop experiment by Robert A. Millikan and Harvey Fletcher in 1909.
Harvey Fletcher was a graduate student of Millikan. Millikan took full credit for the experiment from Fletcher; in return, Fletcher claimed full authorship of a related result for his thesis dissertation. Millikan was awarded the Nobel prize in physics in 1923. Fletcher kept the agreement a secret until his death.
Millikan’s Oil Drop Experiment
Millikan’s oil drop experiment determined the charge of an electron. The experimental setup consists of an atomiser (an oil spray which produces very fine oil droplets), two metal plates, a travelling microscope, a high-voltage power supply, an x-ray source and a container/cover.
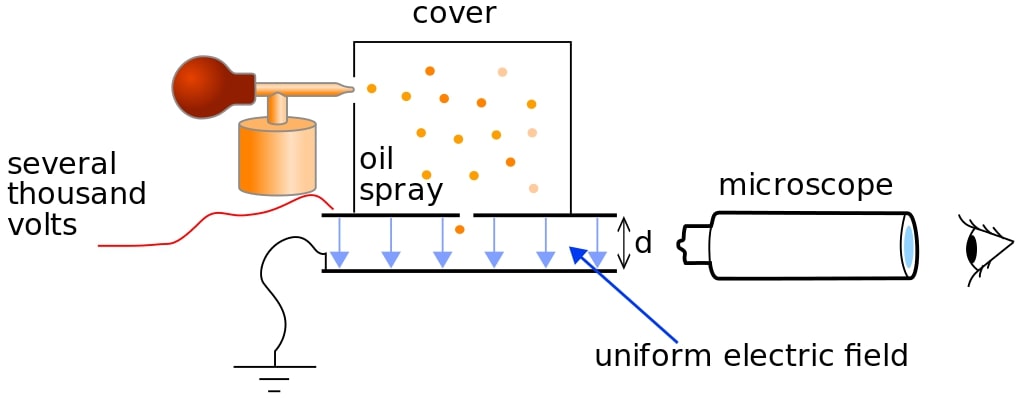
The atomiser sprays very fine oil droplets into the container, where they are ionised by X-Ray. The droplets acquire charges as a result of this ionisation.
Different droplets acquire different charges. The droplets start falling under the effect of gravity and attain terminal velocity. At terminal velocity, the force of viscosity and the buoyant force is equal to the gravitational force.
\( F_g = F_{visc} +F_{buoyant} \) \( m_{drop} g = 6 \pi r \eta v + m_{air} g \)where
\(m_{drop} \) is the mass of an oil drop,
\(m_{air} \) is the mass of air displaced by the oil drop
\( r \) is the radius of a drop
\(\eta \) is the viscosity of air
\(v \) is the terminal velocity.
We assume that the drops are spherical. So, we have,
\( \frac{4}{3} \pi ( \rho_{oil} – \rho ) r^3 g $ = 6 \pi r \eta v \)The terminal velocity of the drop is found by observation through the travelling microscope. Hence, in this way, the radius of the drop is found.
Now, the electric field on the metal plates is turned on. The field is adjusted such that the charged drop stays suspended at a place in the air. When this happens, the viscous force becomes zero due to the zero velocity. So to balance the force, an equal force is applied through the electric force.
\( qE = 6 \pi r \eta v \)The value of q is found to be a small integer multiple of ‘e’, the value of the charge of an electron.
\( e = – 1.60217 \ \times \ 10^{-19} \ C\)FAQs
An electron has a negative charge of magnitude $ 1.60217 \ \times \ 10^{-19} \ C $.
The charge of an electron was first determined using Millikan’s oil drop experiment by Robert A. Millikan and Harvey Fletcher in 1909.
The charge-to-mass ratio of an electron was determined by J. J. Thomson with the cathode ray experiment. Before J. J. Thomson, Cathode ray experiments were carried out by Sir Arthur Schuster. It was J. J. Thomson who identified that the cathode rays are beams of particles called electrons. For this discovery, he was awarded the Nobel prize in 1906. He calculated the charge-to-mass ratio of electrons to good precision in the cathode ray experiment.
Oil does not easily tend to evaporate. If water droplets were used in the experiment, the drops would evaporate. That is why oil is a better choice among the two.
Also Read:
References:
