Phenolphthalein also abbreviated as phph is an organic compound used in acid-base titration as an indicator. This is because of the variation in colours with respect to the change in pH.
phph as a pH indicator shows:
- Pink to red color in alkaline medium, above 8.5 pH.
- Colorless in acidic medium, below 8.5 pH.
It was discovered by Adolf von Baeyer in 1871.
Index
Structure, Formula & Properties
IUPAC Name – 3,3-bis(4-hydroxyphenyl)-2-benzofuran-1-one
Molecular Formula – \(C_{20}H_{14}O_4\)
Molecular Weight – \(318.32 g mol^{-1}\)
Density – \(1.277 g cm^{-3}\)
Melting Point – \(258 – 263^{\circ}C\).
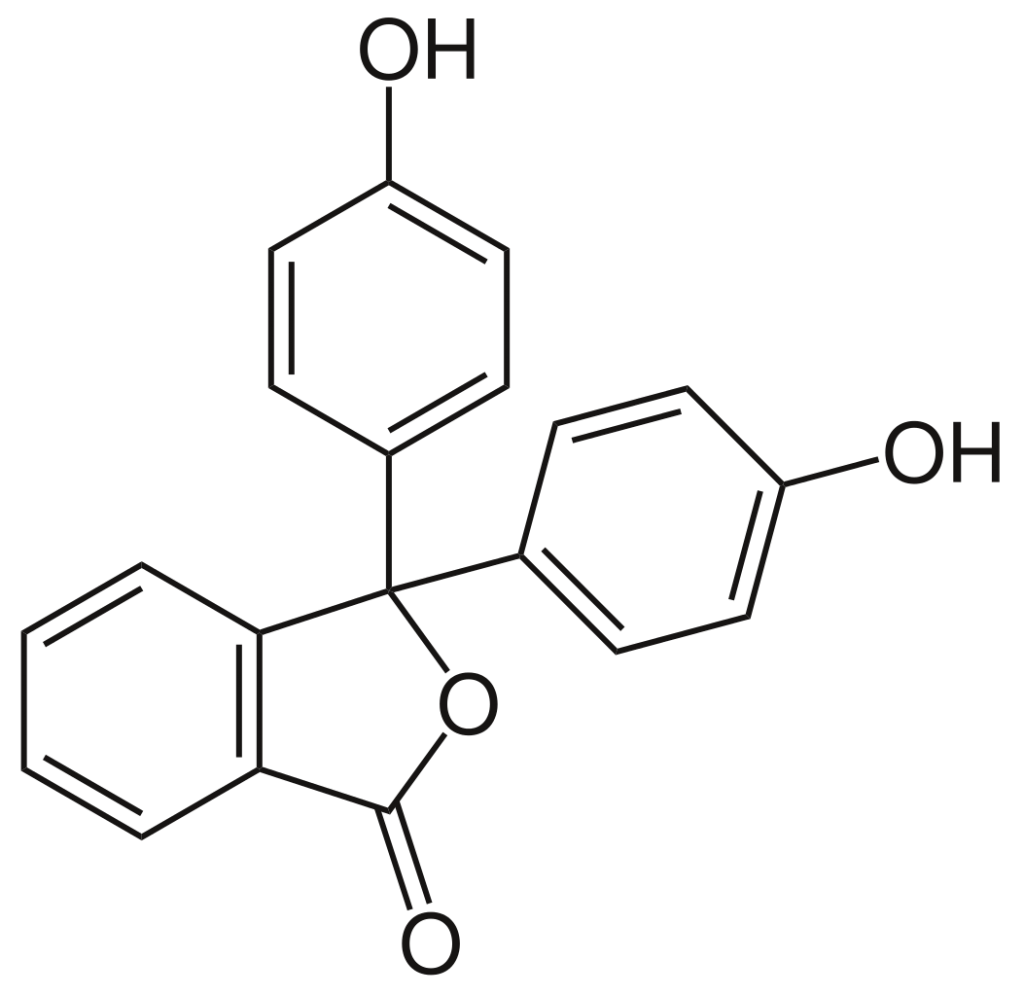
Phenolphthalein is synthesized by phthalic anhydride condensation, with two phenol equivalents under acidic conditions.
Applications
- The compound is popularly used in acid-base titration as an indicator.
- It is also used in various medical tests.
- It is also used in Cement Carbonation.
Related Topic on pH indicator:
1. Methyl Orange
2. Thymolphthalein
FAQs
Since Sodium(Na) and Potassium(K) are metals with basic oxides, which can turn phph solution pink.
phph is colourless in acid.
phph colour varies from pink to red in an alkaline substance.
