Water is the essential component of any living form on Earth. Therefore, it becomes important to study the nature of water for any sane study of life and organisms. Water is a special molecule that has some peculiar and unique phenomena linked to it. One such unusual phenomenon is the Anomalous Thermal Expansion of Water in the temperature range of 0°C to 4°C.
Index
What is Anomalous Expansion of Water?
Most liquids expand upon heating and contract upon cooling. Water, between a specific temperature range, seems to break this notion.
“The Anomalous Expansion of Water is the increase in the volume of water when cooled from 4°C to 0°C (or 39.2°F to 32°F ).”
The figure given below shows a plot of the density vs temperature of the water.
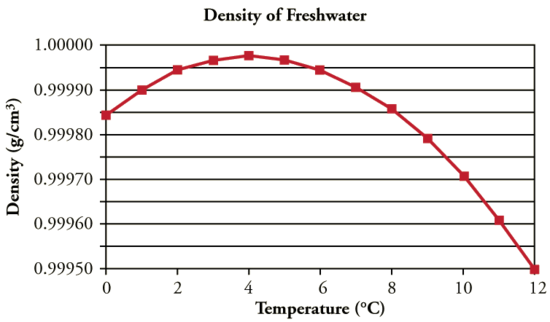
The density of water increases from 0°C to maximum at a temperature of 4°C. After 4°C, it decreases, similar to any other normal behaviour liquid.
Anomalous Expansion happens in the 0°C – 4°C range. In this, the density of water decreases, hence the volume of water increases.
It is also clear from the plot that the density of water is maximum at 4°C with a value of 0.9998395 g/cm3 ~ 1g/cm3.
Reason for Anomalous Expansion of Water
Ice has an “open” crystal structure which has a lot of space. Ice is less dense than water. When ice melts at 0°C, the \(H_2O\) molecules lose this open structure and are more hydrogen bonding. This results in a lesser intermolecular distance between \(H_2O\) molecules.
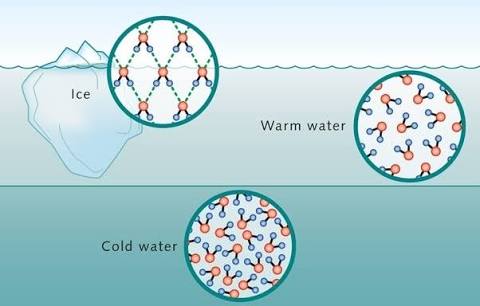
Hence, density increases from 0°C to 4°C. At 4°C, the density is maximum after 4°C, a rise in temperature results in increased intermolecular distance due to an increase in Kinetic Energy of the molecules.
Consequences and Applications
- Preservation of Aquatic Ecosystem:
Anomalous expansion of water is important for sustaining aquatic life in cold regions or during winter. During cold weather, the top layer of a water body cools first. The temperature of the top layer drops to 4°C. The top layer then becomes denser than other layers descends to the bottom of the water body.
This process happens continuously to create a temperature gradient. The bottom most layer remains at 4°C. At this 4°C, aquatic life can thrive. The temperature gradually decreases as the depth decreases. The topmost layer is the coldest. Eventually, the top layer freezes, thereby forming an insulating blanket that prevents further freezing to some extent. If there was no anomalous expansion, the waterworks have frozen altogether. - Weathering of Rocks:
Water seeps into the cracks and crevices of rocks. During winter, when the temperature falls below 4°C, the water expands, resulting in hydrostatic pressure on the rock. This produces cracks on the rocks and helps in weathering the rock. - Pipeline Leakage:
Some water pipelines start to leak when the temperature falls below 4°C. This is due to the pressure created by the anomalous expansion of water.
FAQs
The Anomalous Expansion of Water is the increase in the volume of water when cooled from 4°C to 0°C (or 39.2°F to 32°F ).
Fill a plastic bottle fully with water to the brim. Close the mouth of the bottle with a cork. Cool the bottle in a freezer. The cork comes out after some time when the temperature of the water falls.
Gallium, Silicon, Germanium, etc., show anomalous expansion similar to water.
