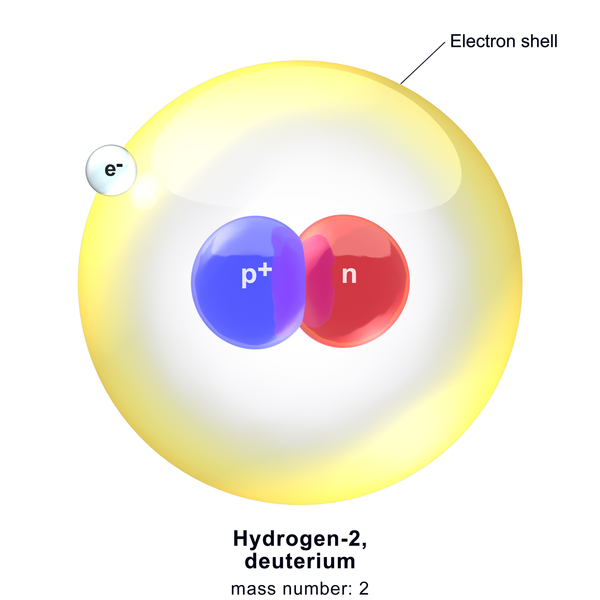
The deuteron is a stable subatomic particle, comprising a proton and a neutron. It is one of the two stable isotopes of hydrogen(H). It is actually the nucleus of a deuterium atom. Deuterium is denoted by D or 2H or Hydrogen-2 and Deuteron is denoted by 2H+.
Before going to learn about deuteron and its mass, let’s have a little knowledge about its history.
Index
History
The name deuterium is derived from the Greek word deuteros, which means “second”. It denotes the two particles composing the nucleus. It was discovered in 1931 by Harold Urey.
The Deuteron and its Mass
The stability of deuteron is remarkable since the free neutron is unstable, undergoing beta decay with a half-life of 10.3 minutes.
| Mass of Deuteron in amu | mD = 2.013553212745(40) u |
| Deuteron mass in MeV | mD = 1875.612 928(12) MeV |
Deuteron has a charge radius of 2.12799 fm.
Deuteron has a charge of +1e.
The binding energy of deuteron has been measured to be 2.2MeV.
Applications
- Deuteron mass can be used to derive the mass of the neutron, which has implications in meteorology.
Hope you enjoyed reading and learning about Deuteron in Atomic theory.
