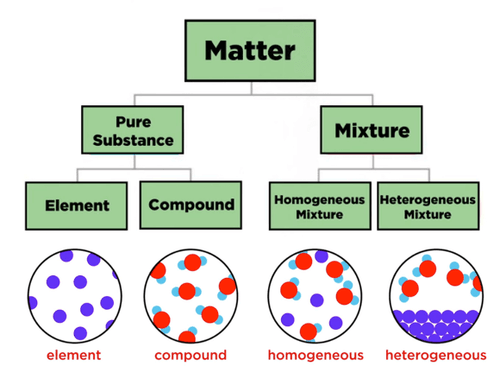
Mixture and compound are common concepts in chemistry. It is important to know their difference in order to be able to distinguish them.
A Video Reference
The table below tells what is the difference between compound and mixture.
Mixture and Compound: Key Difference
| Category | Mixtures | Compounds |
| Definition | Mixtures consist of two or more different types of matter, that can be separated by physical methods. | Compounds consist of two or more chemical elements bonded chemically. They cannot be separated physically. |
| Examples | Salt in water, metal alloys, milk. | Salt crystals, drain cleaner (sodium hydroxide), water |
| Nature | Mixtures can be homogeneous (same composition) or heterogeneous (varying composition). | Compounds are always homogenous. |
| Constituents | The constituents of mixtures can be compounds or elements. | Only elements are the constituents of compounds |
| Ratios | Mixtures have varying ratios of constituents. | Compounds have a fixed ratio of constituents. |
| Separation | Mixtures can be separated by physical methods of separation. | Compounds can be separated only by chemical or electrochemical methods of separation. |
| Properties | The properties of a mixture reflect the properties of its constituents. E.g.: Salt in water. It still is a liquid, like water, and has a salty taste, like salt. | The properties of a compound can be very different from the properties of its constituents. E.g.: Water, H2O, consists of Hydrogen (H) and Oxygen (O), both of which can cause fires. But water puts out fires. |
| New substance formed? | No new substance is formed while a mixture is made, all constituents retain identity. | In the making of a compound, a new substance is made, usually with different properties. |
| Physical properties. | Physical properties like melting point, boiling point and density vary. | Physical properties are fixed. |
FAQs
A mixture is a substance that consists of two or more components that can be separated by physical means. An example of a mixture is Salt in Water.
A compound is a substance that consists of two or more components that can be separated only by chemical or electrochemical means. Example: Water (H2O).
Air is a mixture of many gases like Nitrogen, Oxygen, Carbon Dioxide, etc.
Elements consist of only one type of atom. Examples are nitrogen gas (N2) and oxygen gas (O2).
Compounds consist of two or more types of atoms bonded chemically. They always bond in a fixed ratio, given by the chemical formula. Examples are water (H2O) and table salt (Sodium Chloride, NaCl).
Homogeneous mixtures are mixtures with constant composition throughout. They look like one substance, technically called a phase. Example: Salt in water. Salt is uniformly mixed in the water and present throughout.
Heterogeneous mixtures are mixtures whose composition varies. They are patchy and have regions with different compositions. Example: Oil and water. When oil is mixed in water, it first forms separate drops. After some time, they form separate layers. Each layer is called a phase.
Related Topics:
1. Difference Between Boiling and Evaporation
