Electromeric Effect can be observed in organic compounds that contain at least one multiple bond. It is a transient effect that occurs when the compound is introduced to an attacking reagent.
This effect is a transient effect (a short-lived intermediate effect) that lasts only as long as the attacking reagent is present and in contact with the organic component.
When the attacking reagent is removed from the system, the polarized molecule returns to its natural state.
Index
Mechanism
- Electrons in a pi bond are easily polarized. When charged reagents such as electrophiles or nucleophiles approach, the electrons get polarized and pushed towards one of the atom’s components because of Electrostatic attraction or repulsion.
- The atom that obtains the pair of electrons becomes negatively charged, while the other atom becomes positively charged.
- This movement of electrons from one atom to another is referred to as the electromeric effect.
Types of Electromeric Effect
Based on the direction in which the electron pair is transferred this effect is classified into two types;
+E Electromeric Effect
- +E electromeric effect takes place when the electron pair of the pi bond is shifted towards the attacking reagent.
- The +E effect may be seen when acid is added to alkenes.
- The attacking reagent attaches to the atom that received an electron pair during the transfer.
- When the attacking reagent is an electrophile, the pi electrons are transported towards the positively charged atom, resulting in the +E effect.
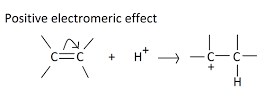
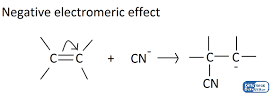
-E Electromeric Effect
- This takes place when the electron pair of the pi bond is pushed away from the attacking reagent.
- The attacking reagent binds to the positively charged atom in the molecule, that is, the atom that lost the electron pair during the transfer.
- When the attacking reagent is a nucleophile, the pi electrons are transported towards the atom with which the attacking reagent will not bond.
Know more: Nucleophile vs Electrophile
Note
The phrase “electromeric effect” is no longer used in standard textbooks and is thought to be obsolete. The resonance effect uses the principles represented by the terms electromeric effect and mesomeric effect. Curved arrows, which indicate the electron shift, can be used to show this phenomenon.
FAQs
The electromeric effect is the intramolecular transfer of electrons from a Pi bond to another atom caused by a reagent attack. There are two kinds of this effect;
1. +E effect – A pi-electron is transferred to the atom that is attacked by the reagent in this effect.
2. – E effect- In this case, an electron is transferred to an atom that is not attacked by the reagent.
Whereas, the delocalization of pi electrons between molecules is referred to as resonance.
When two distinct atoms exchange electrons, the shared electron pair is attracted to the more electronegative atom. This causes polarity to be induced. This is an inductive effect. It carries through the carbon chain. The effect is seen in up to four carbon atoms, whereas in the electromeric effect intramolecular electron transfer occurs and at least one multiple bonds should be present.
