Olfactory Indicators are substances with a characteristic odour in a given acidic or basic medium.
These indicators are often used in laboratories to check whether a given solution is a basic or an acidic solution.
Index
Properties of Olfactory Indicators
As mentioned these are substances with different odours in acid and base solutions.
They have the following characteristic properties:
- Substances with change in odour in acidic or basic solutions.
- These indicators have a characteristics odour.
- This odour changes acidic or basic solutions and helps us detect whether a given sample solution is acidic or basic.
E.g., Vanilla extract has a characteristic pleasant smell in an acidic medium but becomes odourless in basic.
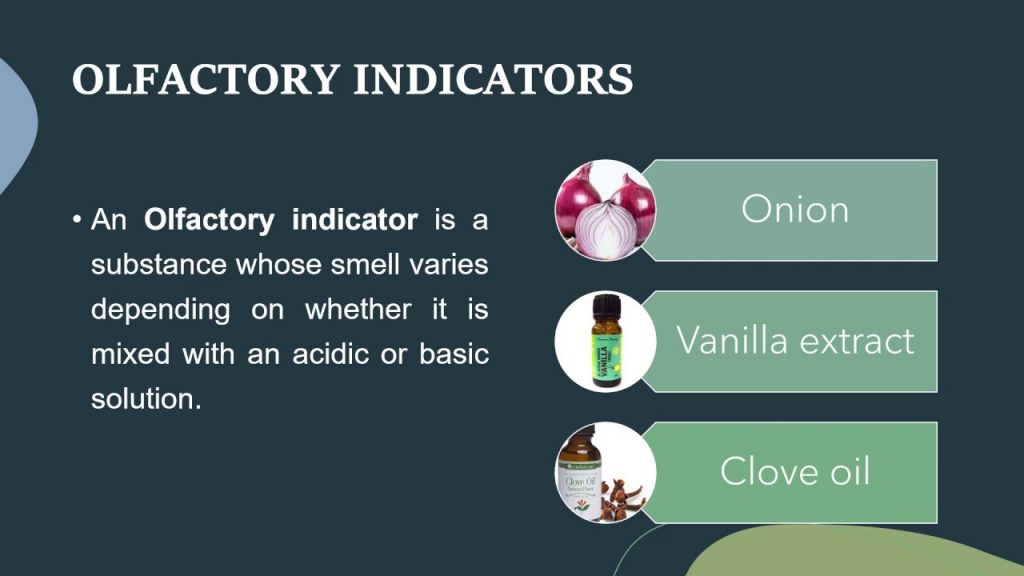
Olfactory Indicator Examples
- Onion
- Retains its smell in an acidic medium
- Loses its smell in basic medium
- Clove oil
- Retains its aroma in an acidic medium
- Loses its smell in basic medium
- Vanilla Extract
- Retains its smell in an acidic medium
- Loses its smell in basic medium
Read more about acids, bases and indicators here: Acids, Bases and Salts.
FAQs
These are substances with a characteristic odour in a given acidic or basic medium. These indicators are often used in laboratories to check whether a given solution is a basic or an acidic solution.
The examples are onion, clove oil and vanilla extract.
We use these in olfactory titration to determine the change of acid to base.
Onion retains its smell in an acidic medium but loses its aroma in a basic medium.
