Electrochemical Series is a way of ordering chemical elements in order of how reactive they are. It uses a concept called Standard Electrode Potential to arrange the elements in the order. It has multiple uses in chemistry.
Index
What is Electrochemical Series?
The electrochemical series is also called the “activity series”. It is a method of arranging elements in order of increasing reactivity. The ordering uses a value called “electrode potential value”.
What is Standard Electrode Potential Value?
Electrode potential value is calculated as follows. An electrode consisting of metal (or non-metal) and its ion is connected to the Standard Hydrogen Electrode (SHE). SHE consists of H2 and H+ ions.
Depending on what metal and ions are used, a characteristic value of voltage is observed across the electrodes, in standard conditions. This is called the “standard electrode potential value” for the given metal/ion combination.
What Does the Electrochemical Series Tell Us?
The electrochemical series tells us how electropositive or electronegative the element/ion combination is, compared to Standard Hydrogen Electrode. This combination is also called a half-cell.
A more electropositive metal loses electrons more easily than hydrogen does in the SHE. On the other hand, a more electronegative element gains electrons more easily.
In general, a more electronegative element takes electrons from a more electropositive one. The electrochemical series thus can be said to be a measure of electronegative nature.
Features of Electrochemical Series
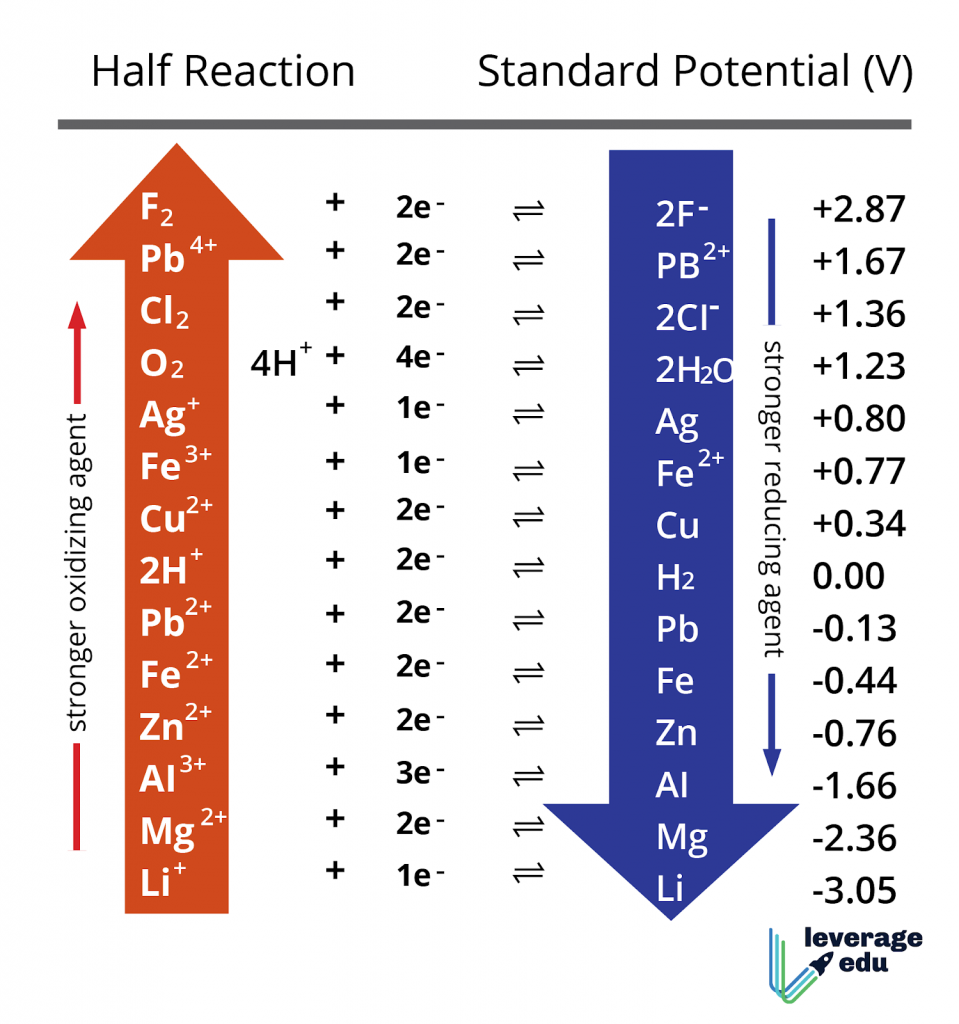
Above is a sample of common elements in the Electrochemical Series. Here are some important features of it:
- Hydrogen (the Standard Hydrogen Potential, SHE) has an electrode potential of 0.00 by definition. All other potentials are defined with respect to it.
- The half-cells (element/ion pairs) with very positive Electrode Potential are high in the Electrochemical Series. They are strong oxidising agents.
- The half-cells with negative electrode potential are reducing agents. The more the reducing power, the more negative is the value.
- Metals are in general are electropositive, while non-metals are electronegative.
- The metals at the bottom are the most reactive. In comparison, the non-metals at the top of the series are the most active. Thus, reactivity is the minimum along the middle.
- Metals higher in the series can be reduced by metals lower in the series. Likewise, non-metals higher in the series can oxidise the metals and nonmetals lower in the series.
Applications of Electrochemical Series
1. Calculation of Cell EMF
Any electrochemical cell consists of two half-cells, at each electrode. Each half cell undergoes a reaction, one is oxidation and the other is reduction. Corresponding to each reaction there is a potential, namely, oxidizing potential and reducing potential.
Cell EMF (\(E^circ_{\text{cell}}\)) is the sum of oxidizing and reducing potentials of the cell. It measures the spontaneity of the overall reaction in the cell. It also is a measure of the work that can be done by the cell.
The electrochemical series helps us measure the cell EMF by taking Standard Electrode Potential values of the half cells, and then adding them suitably.
\(E^\circ_{\text{cell}} = E^\circ_{\text{red}} – E^\circ_{\text{ox}}\)
Here, \(E^\circ_{\text{red}}\) is the Standard Reduction Potential for the reduction half-cell, and \(E^\circ_{\text{ox}}\) is the Standard Reduction Potential for the oxidation half-cell.
2. Measuring Spontaneity of a Reaction
The feasibility or spontaneity of a redox reaction is directly related to the cell EMF of the corresponding reaction.
- If the cell EMF is positive, the reaction is spontaneous.
- If the cell EMF is negative, the reaction is nonspontaneous.
Thus, we can tell if a redox reaction can proceed spontaneously by examining the reactants and products. We write the equations for both reduction and oxidation half-reaction. Then we suitably add their Standard Electrode Potentials based on the electrochemical series.
The resulting cell EMF tells us if the reaction is spontaneous or not.
3. Estimating Gibbs Free Energy
Gibbs free energy (\(\Delta G^\circ_{\text{cell}}\)) is another measure of the spontaneity of a reaction. It is related to the cell EMF (\(E^\circ_{\text{cell}}\)) as follows.
\(\Delta G^\circ_{\text{cell}} = -n F E^\circ_{\text{cell}}\)
Where \(n\) is number of electrons involved in the reaction,
\(F\) is Faraday’s constant, which is equal to \(96,485 \text{ Coulomb}{\text{ mole}}^{-1}\)
Again, based on sign of cell EMF, we have the following:
- If cell EMF is negative, Gibbs Free energy is positive and the reaction is not spontaneous.
- If the cell EMF is positive, Gibbs Free energy is negative, and reaction is spontaneous.
4. Predicting End-Products of a Redox Reaction
If we are given only the reactants of a reaction, we can calculate the end products of the reaction as follows.
We use the electrochemical series to write the Standard Electrode Potential values of each of the reactants. Then, we note which has the highest and lowest reduction potential.
Once we have these values, we can predict the end-products as follows:
- The ions with highest reduction potential are reduced at the cathode.
- The ions with least reduction potential are oxidized at the anode.
The oxidised and reduced ions give us the end-products of the reaction.
Examples
Question 1. Which of the following is the best oxidizing agent and which is the best reducing agent?
\(\mathrm{Fe}^{2+}, \mathrm{Fe}^{3+}, \mathrm{Pb}^{4+} and \mathrm{Fe}^{2+}\)
Solution. From the values of Standard Electrode Potential from Electrochemical Series, we have that \(\mathrm{Pb}^{4+}\) has the most positive Standard Electrode Potential, thus it is best oxidizing agent.
\(\mathrm{Fe}^{2+}\) has the least Standard Electrode Potential thus \(\mathrm{Fe} |\mathrm{Fe}^{2+}\) is the best reducing agent.
Question 2. Calculate the cell EMF of the following reaction:
\($\mathrm{Fe}^{2+} + \mathrm{Cu} \rightarrow \mathrm{Cu}^{2+} + \mathrm{Fe}\)
Is it a spontaneous reaction?
Solution. Clearly, the reaction consists of the following half-reactions.
\(\mathrm{Fe}^{2+} + 2e^- \rightarrow \mathrm{Fe}\)
\(\mathrm{Cu} \rightarrow \mathrm{Cu}^{2+} + 2e^-\)
From the Electrochemical Series, we have the following:
Standard Reduction potential for \(\mathrm{Fe}^{2+} | \mathrm{Fe}\) reduction half-cell \(E^\circ_{\text{red}} = -0.44 \text{ V}\).
Standard Reduction Potential for \(\mathrm{Cu} | \mathrm{Cu}^{2+}\) oxidation half-cell \(E^\circ_{\text{ox}} = 0.34 \text{ V}\).
Thus, cell EMF is given by,
\(\begin{align}
E^\circ_{\text{cell}} &= E^\circ_{\text{red}} – E^\circ_{\text{ox}} \\
&= -0.44 – 0.34 \text{ V} \\
&= -0.78 \text{ V}
\end{align}
\)
As cell EMF is negative, it is not a spontaneous reaction.
FAQs
Electrochemical series is a way of ordering chemical elements in decreasing order of Standard Electrode Potential. In simple terms, it arranges them in order of their reactivity.
Here is a mnemonic for remembering electrochemical series:
Like King Canute Named Magnificent Altered Zebras For Conquering Nile, Snake Hunter Completed Investigating Silver Mercedes Breakdown Claims Over a Flight.
Like – Li – Lithium
King – K – Potassium
Canute – Ca – Calcium
Named – Na – Potassium
Magnificent – Mg – Magnesium
Altered – Al – Aluminium
Zebras – Zn – Zinc
For – Fe – Iron
Conquering – Co – Cobalt
Nile – Ni – Nickel
Snake – Sn – Tin
Hunter – H – Hydrogen
Completed – Cu – Copper
Investigating – I – Iodine
Silver – Silver
Mercedes – Mercury
Breakdown – Br – Bromine
Claims – Cl – Chlorine
Over – Oxygen
A
Flight – F – Fluorine
This Electrochemical Series trick gives the elements in increasing order of Standard Electrode Potential.
The order of electrochemical series is based on the Standard Electrode Potential of the half-cells, specifically, the Standard Reduction Potential.
The strongest reducing agent in electrochemical series is Lithium, which has Standard Electrode Potential -3.05 V.
The weakest reducing agent in electrochemical series is Fluorine, which has Standard electrode Potential +2.87 V.
