Starch is a polymer which is produced in plants. It is used to store the food produced so that it can be used when necessary, it is also insoluble in most common solvents like alcohol and cold water through it dissolves in hot water.
Cellulose is also a polymer which is found in plants. It is mainly present in the cell walls of a plant as it has high tensile strength and is sturdy; it is also present in wood at around 45%. Today, in this article we are going to look into the major differences between these two polymers, i.e starch vs cellulose.
Index
History
Starch grains of Typha grinded to flour 30000 years ago were found in Europe, and those of sorghum were found in some caves of Ngalu, Mozambique, which were dated as 100000 years old.
When the extraction of pure starch started, it was used for many purposes by various civilizations. Ancient Egyptians used it as glue for papyrus, around 77 – 79 AD, Romans used it in cosmetics and also in cooking to thicken sauces, it was also used in cooking by the Persians and Indians and the Chinese used rice starch as the surface treatment in paper production.
The French chemist Anselme Payen discovered Cellulose in 1838 when he isolated it from plant matter. Many important and useful polymers were produced using cellulose in the following years like the Celluloid, the first thermoplastic polymer in 1870, rayon in 1890, and cellophane in 1912.
Hermann Staudinger in 1920 discovered the polymeric structure of cellulose, and it was first artificially produced by Kobayashi and Shoda in 1992.
Read About: Difference Between Cell Wall and Cell Membrane
Monomer Structure of Starch Vs Cellulose
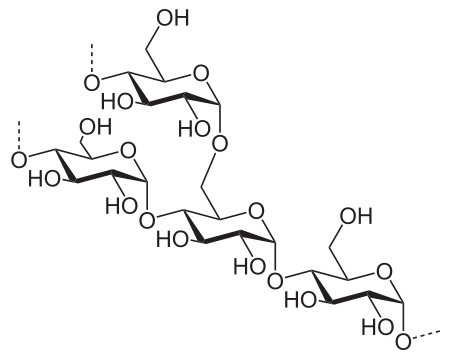
The monomer unit in starch is C6H10O5. The starch polymer is of two types namely
1. Branched (Amylopectin) and
2. Linear (Amylose).
Starch is stored in various parts depending on the plant, sometimes roots and sometimes leaves.
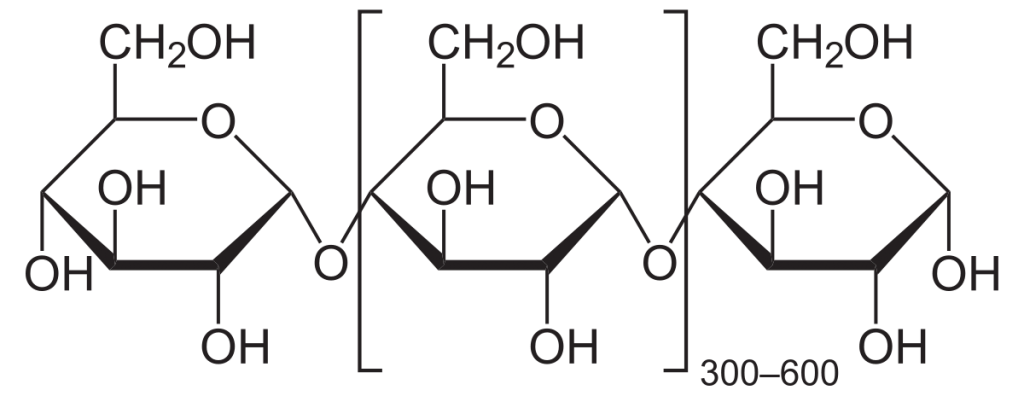
The monomer of cellulose is C6H10O5, i.e, the same as that of starch, but it is a very long linear chain unlike starch, which makes its properties also different.
Presently cellulose is considered the most abundant organic polymer on Earth.
Starch Vs Cellulose
Now, after having an understanding of what starch and cellulose are. Let us look into the major differences between these two polymers, i.e starch vs cellulose.
Physical Properties
Starch
Starch in its pure form is a white tasteless and odourless powder which is insoluble in most common solvents like alcohol and cold water but is soluble in hot water and ionic liquids, mainly metal chloride salt solutions.
Starch undergoes a process called starch gelatinization when it is heated with enough water. In this process, the viscosity of water increases as small amylose chains form a network to hold water together.
The temperature of gelatinization of starch depends on the amount of water and the amount of amylopectin and amylose present in the mixture, and the amount of water present is inversely proportional to the temperature of gelatinization. When taking place in a mixture of water and ionic liquid their ratio has a great influence on gelatinization.
Cellulose
Cellulose is also a white powder which is devoid of both taste and odor. It is insoluble in water and most organic solvents. Cellulose, unlike starch, dissolves in ionic liquids better when there is less water present in the solution.
Structure
Starch
The monomers of starch are bonded via glycosidic bonds, and there are two types of glycosidic bonds present in starch. α-(1,4) glycosidic bonds and α-(1,6) glycosidic bonds.
Amylose is linear and only has α-(1,4) glycosidic bonds, whereas amylopectin is branched, so it has both α-(1,4) and α-(1,6) glycosidic bonds, α-(1,4) glycosidic bonds for the linear part and α-(1,6) glycosidic bonds for the branches which are present at every 35 – 40 units. Though amylose is a linear polymer, it has been found out that there are a few branches present in it too.
Starch molecules arrange themselves into semi-crystalline granules when present in plants, the size of these granules vary depending on the plant, those in potato starch are bigger than rice starch.
Cellulose
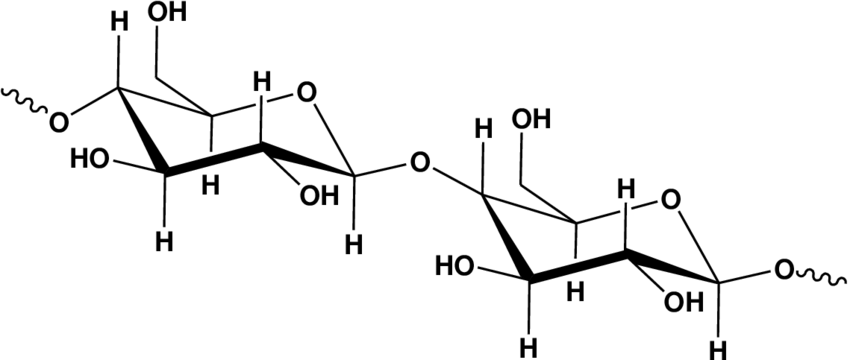
The glycosidic bonds present between monomers of cellulose are β-(1,4) glycosidic bonds. Unlike starch, cellulose is linear and has a straight rod like structure, but the chain length of each molecule present in bacteria and cotton is around 800 to 10,000 units and in wood pulp is 300 to 1700 units.
Due to the β-(1,4) glycosidic bonds present in cellulose, the hydroxyl groups present in it form hydrogen bonds with the same chain or neighboring chains which holds them together firmly making it a strong microfibrils with high tensile strength. Due to the hydrogen bonds present between individual chains cellulose is more crystalline than starch, which results in a higher melting point(320 OC) and more strength than starch.
Chemical Decomposition
Starch
Starch is broken into its constituent sugars through the process of hydrolysis using enzymes called amylases. These enzymes are present in the digestive systems of animals. In the human digestive system, saliva and pancreatic juice have amylase.
Cellulose
Cellulose is broken into cellodextrins or glucose through the process of cellulolysis; these are hydrolysis reactions. Cellulose molecules have hydrogen bonds between themselves, so their breakdown is more difficult than starch molecules. Due to this fact, most animals which have to digest cellulose have separate mechanisms and processes for it.
FAQs
Starch and cellulose are two polymers generally found in plants. They are similar in a few terms and different in a few.
Basically, both are made of the same monomer, glucose, and have the same replicate units based on glucose.
The only major difference is that glucose units in starch are connected by α- linkages whereas these units in cellulose are connected by β- linkages.
Cellulose is stronger than starch mainly due to the way glucose monomers connect in it as it allows for more hydrogen bonds which in turn increases its strength.
