Lindlar catalyst is basically palladium deposited on calcium carbonate or barium sulfate, poisoned with various forms of lead and sulfur. It is a heterogeneous catalyst.
Lindlar catalyst is named after its inventor, a British chemist, Herbert Lindlar.
Index
Synthesis of Lindlar Catalyst
Lindlar catalyst is commercially prepared by reducing palladium chloride in a slurry of calcium carbonate(CaCO3) followed by adding lead acetate. Apart from lead acetate, other catalyst poisons can also be used such as lead oxide and quinoline.
Lindlar Catalyst Properties
Here are some of the properties of Lindlar Catalysts.
| Specific Surface Area | 150 – 260 m2/g |
| Impurity | ≤ 0.5% |
| Water Content | ≤ 5% |
| pH | 8 |
Mechanism
Lindlar catalysts allow the reduction or hydrogenation of the alkyne via syn addition, i.e., the addition of the substituent on the same side of the bond. This leads to a product of the cis-isomer of the alkene.
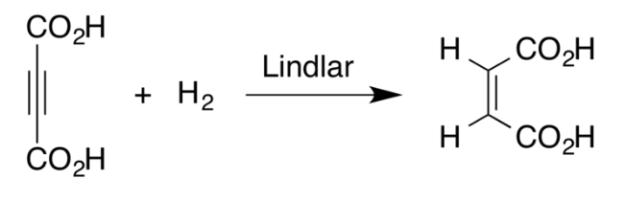
The catalyst is used for the reduction of alkynes to alkenes, without further reduction into alkanes. The lead salt deactivates the palladium sites which prevents the formation of alkanes. Hence, if a compound is containing a double bond as well as a triple bond is hydrogenated using this catalyst, only the triple bond is reduced.
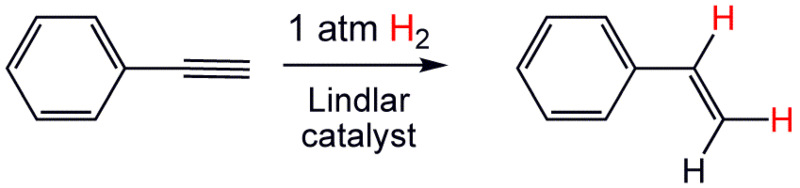
An example of this is the reduction of phenylacetylene to styrene.
Applications of Lindar Catalyst
- Lindlar catalysts are used for the reduction of alkynes to cis-alkenes.

- A commercial application of Lindlar catalyst is the organic synthesis of vitamin A. This synthesis involves an alkyne reduction with the Lindlar catalysts.
FAQs
Palladium catalysts generally have high catalytic activities that may even reduce double bonds. Reduction of alkynes with such catalysts may lead to the formation of alkanes. Thus the strength of the catalyst is reduced by adding or “poisoning” the palladium catalyst with lead salts or quinoline.
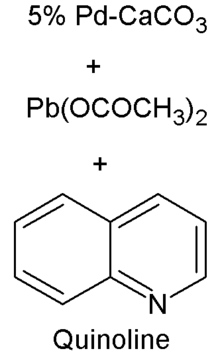
A typical formula is Pd-CaCO3, Pb(OCOCH3)2, and C9H7N(quinoline).
Lindlar catalysts are prepared by reducing palladium chloride in a slurry of calcium carbonate(CaCO3) followed by adding lead acetate. Apart from lead acetate, other catalyst poisons can also be used such as lead oxide and quinoline.
