Decantation is a useful separation technique used to separate mixtures of non-miscible liquids, or mixtures of solids and liquids.
Index
What is Decantation?
Decantation is a method of separation of mixtures. It works for mixtures of immiscible liquids, and also for mixtures of solids and liquids.
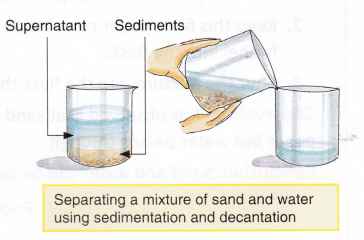
In the process, the mixture is first left undisturbed. If it is a mixture of immiscible liquids, they will form layers. If it is a mixture of liquids and solids, the solids will settle down.
Once this step is achieved, the liquid floating at the top is carefully poured away. Care is taken so that the materials below do not get transferred.
This can work, for example in the case of water and sand mixture. We can pour away the water on top when the sand settles down. This helps separate them.
It can also be used to separate a mixture of oil and water.
Variations in Decantation
Decantation alone might not be sufficient for some mixtures. This is why we discuss variations below, which can improve the process.
Separating Funnel
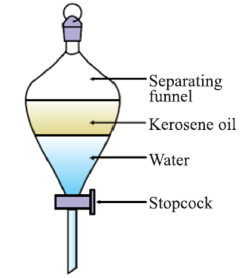
A separating funnel can improve separation efficiency in decantation. This works for mixtures of immiscible liquids. It consists of a small glass container with a stopcock to control flow. We let the components of the mixture settle down like before. We can let the bottom-most layer flow away, keeping the other layers still. Careful usage of the stopcock can ensure best results.
Centrifugation
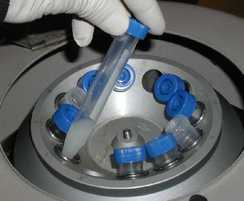
This is often used for solid-liquid mixtures. In the process, a centrifuge is used to forcefully settle down the solid component of the mixture. This forms what is called a precipitate. The rotation of the centrifuge significantly speeds up the settling down process. It can also increase separation quality, as the solid accumulates as a hard pellet that doesn’t mix back with the liquid.
Loading
Sometimes decantation by itself doesn’t fully separate solid-liquid mixtures. If precipitation is slow, we can use centrifugation, as above.
Another way that can speed up the separation of mud and water is called loading. In the process, a mineral called alum is added to the mixture. This attaches itself to the mud particles, making them heavy, and thus settle down faster. This is used in water purification systems.
Applications of Decantation in Daily Life
Here, we shall see some real-life examples of decantation.
- Decantation, along with loading, is often used in water purification.
- Scientific laboratories rely on centrifugation and decantation for separation of a variety of mixtures.
- In medicine, this separation technique is used to separate blood cells and plasma.
- The food industry has several uses for decantation:
- Cream and butter are separated from milk using this technique.
- In sugar making, multiple steps of decantation are used to arrive at sugar crystals at the end.
- This technique is a vital step in processing of vinegar too.
- It is used in removal of unnecessary byproducts like glycerine in biodiesel production.
- When petroleum needs to be separated from sea water, decantation is used. This is because petroleum is lighter than water, and floats on it.
FAQs
Decantation is a method of separation of mixtures of solid and liquid, or two or more immiscible liquids. The mixture is allowed to settle down into multiple layers, and the lightest/heaviest liquid layers are poured off.
Examples of this technique include:
1. Separation of mud-water mixture (solid-liquid mixture)
2. Separation of oil and water (immiscible liquid mixture)
3. Separation of kerosene and water.
We can use this separation technique to separate two kinds of mixtures:
1. Solid-liquid mixtures (mud in water, blood cells in plasma, etc)
2. Liquid-liquid mixtures (the liquids must be immiscible) such as oil and water, petrol and water.
In filtration, a physical filter is used to pass the mixture through. The liquid passes through, and the solid is stopped by the filter.
In decantation, the mixture is allowed to settle down into layers, then the topmost layer is poured off. Alternatively, the bottom-most layer can be separated by using a stopcock.
The topmost layer of liquid that is poured off in this separation technique is called the supernatant.
